05091
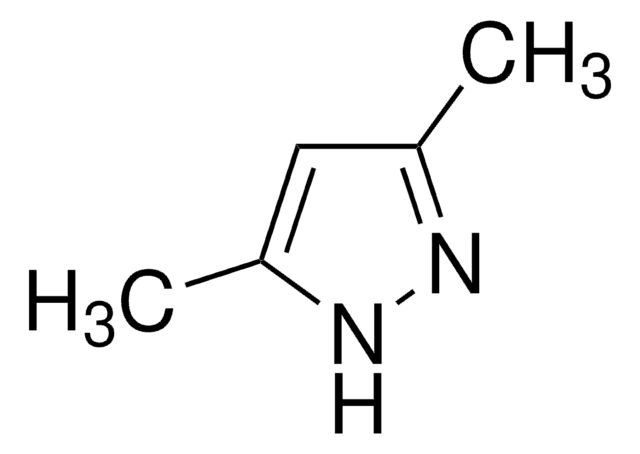
3,5-Dimethylpyrazole
produced by Wacker Chemie AG, Burghausen, Germany, ≥99.0% (GC)
Synonym(s):
3,5-Dimethyl-1H-pyrazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8N2
CAS Number:
Molecular Weight:
96.13
Beilstein:
106325
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39160503
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
produced by Wacker Chemie AG, Burghausen, Germany
Quality Level
Assay
≥99.0% (GC)
bp
218 °C (lit.)
mp
105-108 °C (lit.)
SMILES string
Cc1cc(C)[nH]n1
InChI
1S/C5H8N2/c1-4-3-5(2)7-6-4/h3H,1-2H3,(H,6,7)
InChI key
SDXAWLJRERMRKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - STOT RE 2
Target Organs
Liver
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alessandra Del Roso et al.
Experimental gerontology, 38(5), 519-527 (2003-05-14)
Autophagy is a universal, highly regulated mechanism responsible for the degradation of long-lived proteins, cytomembranes and organelles during fasting and may be the cell repair mechanism that mediates the anti-ageing effects of calorie restriction (Bergamini and Gori, 1995). The function
E Bergamini et al.
The American journal of physiology, 266(1 Pt 1), G118-G122 (1994-01-01)
Regulation of liver macroautophagy and protein degradation by hormones and direct regulatory amino acids were studied in male 2-mo-old Sprague-Dawley albino rats with the use of the antilipolytic agent 3,5'-dimethylpyrazole (DMP; 12 mg/kg body wt ip) as a stimulatory agent.
Rupam Sarma et al.
Dalton transactions (Cambridge, England : 2003), (36)(36), 7428-7436 (2009-09-04)
The reactions of 3,5-dimethylpyrazole with zinc(II)acetate dihydrate and varieties of aromatic carboxylic acids led to formation of mono-nuclear zinc complexes of composition [Zn(HDMP)2(RCO2)2] (R = C6H5, p-CH3-C6H4, p-NO2-C6H4 etc. HDMP = 3,5-dimethylpyrazole) in methanol, whereas the same reactants in dimethylformamide
Sara Straniero et al.
Rejuvenation research, 12(2), 77-84 (2009-05-08)
Aging is characterized by several metabolic changes responsible for the decline of certain functions and the appearance of age-related diseases, including hypercholesterolemia, which is the main risk factor for atherosclerosis and cardiovascular disease. Similar changes in a number of morphological
T Locci Cubeddu et al.
Biochimica et biophysica acta, 839(1), 96-104 (1985-03-29)
The mechanisms involved in the inhibitory effects of antilipolytic agents on rat liver peroxisomal fatty acid oxidative activity have been explored. Treatment of fasting rats with antilipolytic drugs (either 3,5-dimethylpyrazole (12 mg/kg body weight) or Acipimox (25 mg/kg body weight]
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service