All Photos(1)
About This Item
Empirical Formula (Hill Notation):
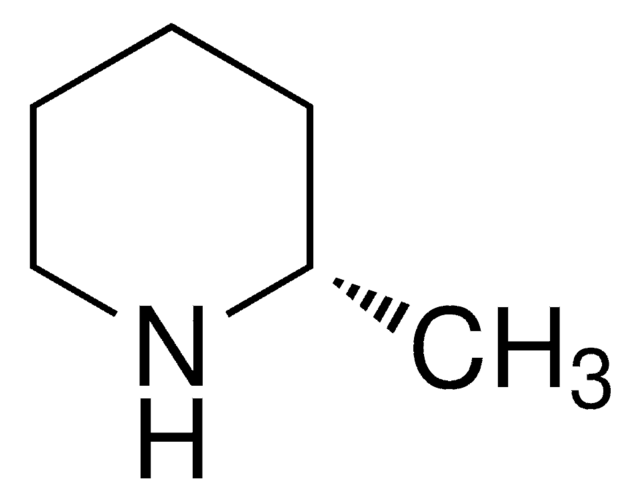
C5H11N
CAS Number:
Molecular Weight:
85.15
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.4353
Quality Level
density
0.842 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[C@@H]1CCCN1
InChI
1S/C5H11N/c1-5-3-2-4-6-5/h5-6H,2-4H2,1H3/t5-/m1/s1
InChI key
RGHPCLZJAFCTIK-RXMQYKEDSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(R)-(−)-2-Methylpyrrolidine, an optically active amine that can be used as a key building block to synthesize:
- 4,5-fused pyridazinone derivatives are applicable as potent histamine H3 receptor antagonists.
- Naphthalenoid histamine H3 receptor antagonist.
- Pyrrolo[2,3-d]pyrimidine derivatives as potent inhibitors of leucine-rich repeat kinase 2.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
45.0 °F - closed cup
Flash Point(C)
7.22 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
In vitro SAR of pyrrolidine-containing histamine H 3 receptor antagonists: Trends across multiple chemical series
Nersesian, Diana L., et al.
Bioorganic & Medicinal Chemistry Letters, 18.1, 355-359 (2008)
Optical Activation of Racemic a-Substituted Carbonyl Compounds Using Optically Active Amines.
Matsushita H, et al.
Bulletin of the Chemical Society of Japan, 49(7), 1928-1930 (1976)
An expedient and multikilogram synthesis of a naphthalenoid H3 antagonist.
Pu YM, et al.
Organic Process Research & Development, 11(6), 1004-1009 (2007)
Ming Tao et al.
Bioorganic & medicinal chemistry letters, 21(20), 6126-6130 (2011-09-13)
Three series of novel 4,5-fused pyridazinones were synthesized as histamine H(3) receptor antagonists. The 2,5,6,7-tetrahydrocyclopenta[d]pyridazin-1-one 5q and 5,6,7,8-tetrahydro-2H-phthalazin-1-one 5u displayed high affinity at both rat and human H(3) receptors, and showed potent antagonist and full inverse agonist activity in functional
Douglas S Williamson et al.
Journal of medicinal chemistry, 64(14), 10312-10332 (2021-06-30)
Inhibitors of leucine-rich repeat kinase 2 (LRRK2) and mutants, such as G2019S, have potential utility in Parkinson's disease treatment. Fragment hit-derived pyrrolo[2,3-d]pyrimidines underwent optimization using X-ray structures of LRRK2 kinase domain surrogates, based on checkpoint kinase 1 (CHK1) and a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service