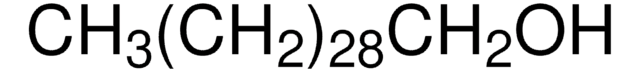All Photos(1)
About This Item
Linear Formula:
CH3(CH2)21OH
CAS Number:
Molecular Weight:
326.60
Beilstein:
1770470
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
180 °C/0.22 mmHg (lit.)
mp
65-72 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCCCCCCCCO
InChI
1S/C22H46O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23/h23H,2-22H2,1H3
InChI key
NOPFSRXAKWQILS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Docosanol inhibits replication of certain viruses (herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. It has been isolated from Clematis brevicaudata.
Application
1-Docosanol was used in the synthesis of series of amphiphilic dendrimers with hydrophilic aliphatic polyether-type dendritic core and hydrophobic docosyl peripheries.
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
410.0 °F
Flash Point(C)
210 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ai-Mei Yang et al.
Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 32(10), 1534-1537 (2010-02-02)
To study the chemical constituents from Clematis brevicaudata. The compounds were isolated by column chromatography and their structures were elucidated through spectroscopic analysis (NMR). Eight compounds were isolated and identified as: palmitic acid (1), 1-docosanol (2), pentacosanoic acid-2', 3'-dihydroxypropyl ester
John J Docherty et al.
Antiviral research, 61(1), 19-26 (2003-12-13)
Resveratrol (3,5,4'-trihydroxystilbene) is a natural component of certain foods, such as grapes, that has been shown to have anti-herpes simplex virus (HSV) activity in vitro. To determine if it is active in vivo, the abraded epidermis of SKH1 mice were
Xiuli Yue et al.
The journal of physical chemistry. B, 110(44), 22237-22244 (2006-11-03)
A systematic analysis of pressure-area isotherms and grazing incidence X-ray diffraction (GIXD) data of 22-methoxydocosan-1-ol (H3C-O-(CH2)22-OH, MDO), docosan-1-ol (H3C-(CH2)21-OH, DO), and docosyl methyl ether (H3C-(CH2)21-O-CH3, DME) monolayers on pure water between 10 and 35 degrees C is presented. All monolayers
Luciana María Cocchiararo-Bastias et al.
Journal of chemical ecology, 37(3), 246-252 (2011-03-05)
Epicuticular lipids are contact cues in intraspecific chemical communication in insects, both for aggregation and sexual behavior. Triatomine bugs are vectors of the parasite Trypanosoma cruzi, the cause of Chagas disease. In Triatoma infestans, the major epicuticular lipids are hydrocarbons
Daniel T Leung et al.
Expert opinion on pharmacotherapy, 5(12), 2567-2571 (2004-12-02)
Recurrent herpes labialis is a painful and potentially disfiguring infection affecting an estimated 40 million people in the US alone. The majority of recurrences are caused by herpes simplex virus type 1. Various oral and topical formulations of nucleoside analogues
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service