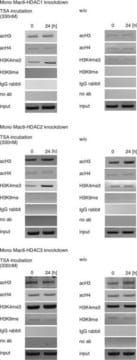
MAB3404
Anti-Cytokeratin 18 Antibody, clone CK2
clone CK2, Chemicon®, from mouse
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
CK2, monoclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
immunocytochemistry: suitable
immunohistochemistry: suitable
isotype
IgG1
NCBI accession no.
UniProt accession no.
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
human ... KRT18(3875)
Specificity
The antibody reacts with cytokeratin No. 18 from human. This antibody is used for staining research samples of a wide variety of simple epithelia and simple glandular epithelia (e. g. intestinal, respiratory and urinary epithelia) and transitional epithelia (e. g. bladder), but stratified squamous epithelia are not stained (e. g. esophagus, epidermis). Keratin filaments of cell lines such as HeLa cells are stained (Debus et al., 1982). For staining research samples of human tumor material with this antibody, see (Debus et al., 1984). For the cytokeratin pattern of different tissues, see (Moll et al., 1982).
Immunogen
Cytoskeletal preparation of HeLa cells (Debus et al., 1982).
Application
Immunocytochemistry: 20 μg/mL
Immunohistochemistry: 20 μg/mL Not for formaldehyde fixed tissues.
Optimal working dilutions must be determined by end user.
Immunohistochemistry/Immunocytochemistry Protocols:
Ideal specimens are obtained from frozen sections from shockfrozen tissue samples. The frozen sections are dried in the air and then fixed with acetone at -15 to -25°C for 10 min. Excess acetone is allowed to evaporate at 15-25°C. Material fixed in alcohol and embedded in paraffin can, however, be used, see (4). For the immunohistochemical detection of cytokeratin No. 18 in tissue sections, the tissue should not be fixed in formaldehyde as this fixative markedly reduces the staining intensity of the cytokeratin filaments. lt is advantageous to block unspecific binding sites by overlaying the sections with fetal calf serum for 20-30 min at 15-25°C. Excess of fetal calf serum is removed by decanting before application of the antibody solution.
Cytocentrifuge preparations of single cells or cell smears are also fixed in acetone. These preparations should, however, not be dried in the air. Instead, the excess acetone is removed by briefly washing in phosphate-buffered saline (PBS). Further treatment is then as follows:
• Overlay the preparation with 10-20 μL antibody solution and incubate in a humid chamber at 37°C for 1h.
• Dip the slide briefly in PBS and then wash 3 times in PBS for 3 min (using a fresh PBS bath in each case).
• Wipe the margins of the preparation dry and overlay the preparation with 10-20 μL of an anti-mouse-IgG-FITC or anti-mouse-IgG-peroxidase antibody and allow to incubate for 1 h at 37°C in a humid chamber.
• Wash the slide as described above. The preparation must not be allowed to dry out during any of the steps. lf using an indirect immunofluorescence technique, the preparation should be overlaid with a suitable embed-ding medium (e. g. Moviol, Hoechst) and examined under the fluorescence microscope.
lf a POD-conjugate has been used as the secondary antibody, the preparation should be overlaid with a substrate solution (see below). and incubated at 15-25°C until a clearly visible redbrown color develops. A negative control (e. g. only the secondary antibody) should remain unchanged in color during this incubation period.
Wash away the substrate with PBS and stain the preparation, if desired, with hemalum stain, for about 1 min. The hemalum solution is washed off with PBS, the peparation is embedded and examined.
Substrate solutions:
Aminoethyl-carbazole Dissolve 2mg 3-amino-9-ethylcarbazole with 1.2 mL dimethylsulfoxide and add 28.8 mL Tris-HCI, 0.05 M; pH 7.3, and 20 μL 3% H 2 O 2 (w/v). Prepare solution freshly each day. Diaminobenzidine Dissolve 25 mg 3,3′-diaminobenzidine with 50 ml Tris-HCI, 0.05 M; pH 7.3, and add 40 μL H 2 O 2 , 3% (w/v). Prepare solution freshly each day.
Immunohistochemistry: 20 μg/mL Not for formaldehyde fixed tissues.
Optimal working dilutions must be determined by end user.
Immunohistochemistry/Immunocytochemistry Protocols:
Ideal specimens are obtained from frozen sections from shockfrozen tissue samples. The frozen sections are dried in the air and then fixed with acetone at -15 to -25°C for 10 min. Excess acetone is allowed to evaporate at 15-25°C. Material fixed in alcohol and embedded in paraffin can, however, be used, see (4). For the immunohistochemical detection of cytokeratin No. 18 in tissue sections, the tissue should not be fixed in formaldehyde as this fixative markedly reduces the staining intensity of the cytokeratin filaments. lt is advantageous to block unspecific binding sites by overlaying the sections with fetal calf serum for 20-30 min at 15-25°C. Excess of fetal calf serum is removed by decanting before application of the antibody solution.
Cytocentrifuge preparations of single cells or cell smears are also fixed in acetone. These preparations should, however, not be dried in the air. Instead, the excess acetone is removed by briefly washing in phosphate-buffered saline (PBS). Further treatment is then as follows:
• Overlay the preparation with 10-20 μL antibody solution and incubate in a humid chamber at 37°C for 1h.
• Dip the slide briefly in PBS and then wash 3 times in PBS for 3 min (using a fresh PBS bath in each case).
• Wipe the margins of the preparation dry and overlay the preparation with 10-20 μL of an anti-mouse-IgG-FITC or anti-mouse-IgG-peroxidase antibody and allow to incubate for 1 h at 37°C in a humid chamber.
• Wash the slide as described above. The preparation must not be allowed to dry out during any of the steps. lf using an indirect immunofluorescence technique, the preparation should be overlaid with a suitable embed-ding medium (e. g. Moviol, Hoechst) and examined under the fluorescence microscope.
lf a POD-conjugate has been used as the secondary antibody, the preparation should be overlaid with a substrate solution (see below). and incubated at 15-25°C until a clearly visible redbrown color develops. A negative control (e. g. only the secondary antibody) should remain unchanged in color during this incubation period.
Wash away the substrate with PBS and stain the preparation, if desired, with hemalum stain, for about 1 min. The hemalum solution is washed off with PBS, the peparation is embedded and examined.
Substrate solutions:
Aminoethyl-carbazole Dissolve 2mg 3-amino-9-ethylcarbazole with 1.2 mL dimethylsulfoxide and add 28.8 mL Tris-HCI, 0.05 M; pH 7.3, and 20 μL 3% H 2 O 2 (w/v). Prepare solution freshly each day. Diaminobenzidine Dissolve 25 mg 3,3′-diaminobenzidine with 50 ml Tris-HCI, 0.05 M; pH 7.3, and add 40 μL H 2 O 2 , 3% (w/v). Prepare solution freshly each day.
This Anti-Cytokeratin 18 Antibody, clone CK2 is validated for use in IC, IH for the detection of Cytokeratin 18.
Linkage
Replaces: 04-586
Physical form
Format: Purified
Storage and Stability
The lyophilized antibody is stable when stored at -20°C.Store the reconstituted antibody solution at 2-8°C, for up to 12 months.DO NOT FREEZE.
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T S Weiss et al.
Gut, 57(8), 1129-1138 (2008-04-18)
Liver regeneration is mainly based on cellular self-renewal including progenitor cells. Efforts have been made to harness this potential for cell transplantation, but shortage of hepatocytes and premature differentiated progenitor cells from extra-hepatic organs are limiting factors. Histological studies implied
Amber Leah Jolly et al.
mBio, 10(2) (2019-03-21)
Chlamydia trachomatis ocular strains cause a blinding disease known as trachoma. These strains rarely cause urogenital infections and are not found in the upper genital tract or rectum. Urogenital strains are responsible for a self-limited conjunctivitis and the sequelae of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service