All Photos(1)
About This Item
Empirical Formula (Hill Notation):
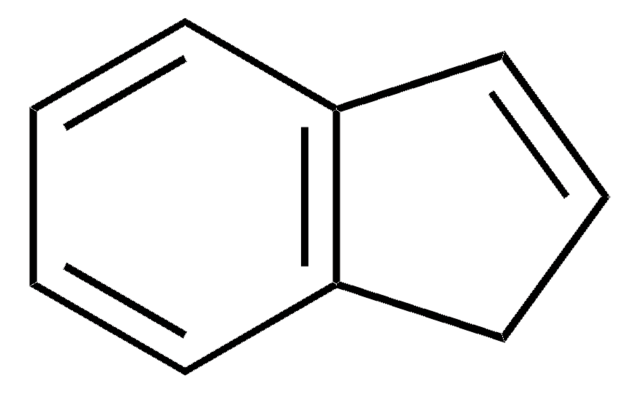
C9H10
CAS Number:
Molecular Weight:
118.18
Beilstein:
1904376
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.537 (lit.)
bp
176 °C (lit.)
mp
−51 °C (lit.)
density
0.965 g/mL at 25 °C (lit.)
SMILES string
C1Cc2ccccc2C1
InChI
1S/C9H10/c1-2-5-9-7-3-6-8(9)4-1/h1-2,4-5H,3,6-7H2
InChI key
PQNFLJBBNBOBRQ-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wei Li et al.
Chemical communications (Cambridge, England), 46(22), 3979-3981 (2010-04-22)
(S,R)-Indan-ambox ligand and its ruthenium(II) complex have been prepared and successfully applied to asymmetric hydrogenation of prochiral simple ketones. A wide range of unfunctionalized ketones are reduced by Ru(II)-indan-ambox catalyst with excellent enantioselectivities (up to 97% ee).
Kenji Kabashima et al.
FEBS letters, 578(1-2), 36-40 (2004-12-08)
Nuclear factor kappa B (NF-kappaB) plays a wide variety of pathophysiological roles and modulation of its pathway can be a good novel drug target. Here, we found that our recently synthesized NF-kappaB inhibitor attenuated an ovalbumin-specific delayed-type hypersensitivity response in
Sarathy Kesavan et al.
Organic letters, 9(25), 5203-5206 (2007-11-14)
Alkylidene indane and ring-expanded scaffolds have been prepared using an enantioselective crotylation/Heck cyclization sequence. Further diversification using consecutive cyclopropanation-Cope rearrangement affords novel chemotypes including spiroindane frameworks.
Naoki Tarui et al.
Bioscience, biotechnology, and biochemistry, 66(2), 464-466 (2002-05-10)
Racemic indan derivatives have been resolved by the hydrolysis of amide bonds using Corynebacterium ammoniagenes IFO12612 to produce (S)-amine and (R)-amides. In the kinetic resolution of 1 (N-12-(6-methoxy-indan-1-yl)ethyl]acetamide), it was possible to run the reaction to 44% conversion on a
Peter R Andreana et al.
Organic letters, 6(23), 4231-4233 (2004-11-05)
A catalytic asymmetric Passerini reaction using tridentate indan (pybox) Cu(II) Lewis acid complex 4 with substrates capable of bidentate coordination has been achieved. The reaction occurs via ligand-accelerated catalysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service