A78608
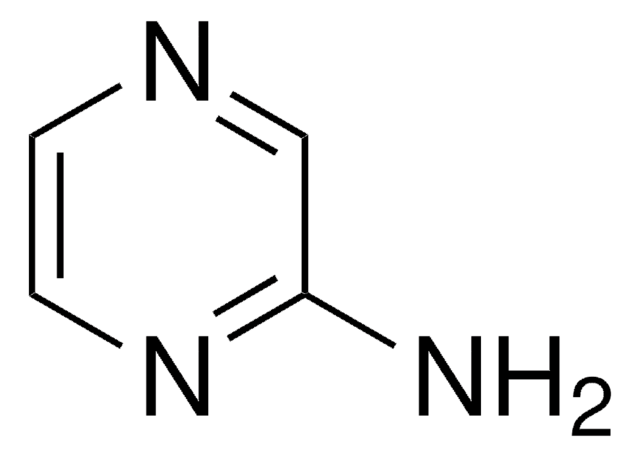
2-Aminopyrimidine
97%
Synonym(s):
2-Pyrimidinamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5N3
CAS Number:
Molecular Weight:
95.10
Beilstein:
107014
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
122-126 °C (lit.)
SMILES string
Nc1ncccn1
InChI
1S/C4H5N3/c5-4-6-2-1-3-7-4/h1-3H,(H2,5,6,7)
InChI key
LJXQPZWIHJMPQQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kamaljit Singh et al.
European journal of medicinal chemistry, 52, 82-97 (2012-03-31)
2-Aminopyrimidine based 4-aminoquinolines were synthesized using an efficacious protocol. Some of the compounds showed in vitro anti-plasmodial activity against drug-sensitive CQ(S) (3D7) and drug-resistant CQ(R) (K1) strains of Plasmodium falciparum in the nM range. In particular, 5-isopropyloxycarbonyl-6-methyl-4-(2-nitrophenyl)-2-[(7-chloroquinolin-4-ylamino)butylamino] pyrimidine depicted the lowest
Robert J Altenbach et al.
Journal of medicinal chemistry, 51(20), 6571-6580 (2008-09-25)
A series of 2-aminopyrimidines was synthesized as ligands of the histamine H4 receptor (H4R). Working in part from a pyrimidine hit that was identified in an HTS campaign, SAR studies were carried out to optimize the potency, which led to
Manishkumar D Joshi et al.
Journal of chromatography. A, 1308, 161-165 (2013-08-21)
A reversed-phase high performance liquid chromatography (HPLC) method is described for the determination of boronic acids that are commonly present as impurities in pinacolboronate ester reagents. Boronic acids and their pinacolboronate esters are key reagents in the Suzuki-Miyaura coupling reaction.
Jinho Lee et al.
Bioorganic & medicinal chemistry letters, 21(14), 4203-4205 (2011-06-21)
A series of new 2-(2-aminopyrimidin-4-yl)phenol derivatives were synthesized as potential antitumor compounds. Substitution with pyrrolidine-3,4-diol at the 4-position of phenol provided potent inhibitory activity against CDK1 and CDK2. X-ray crystal structural studies were performed to account for the effect of
Manuel Tzouros et al.
Chemical research in toxicology, 22(5), 853-862 (2009-03-26)
Covalent binding of reactive metabolites is generally accepted as one underlying mechanism of drug-induced toxicity. However, identification of protein targets by reactive metabolites still remains a challenge due to their low abundance. Here, we report the development of a highly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service