671576
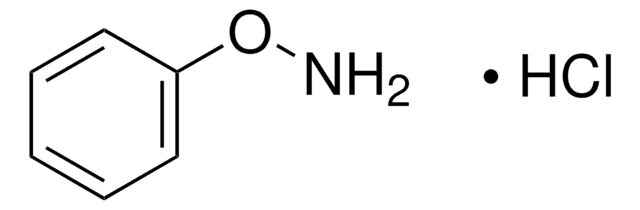
N-Phenylhydroxylamine
≥95.0%
Synonym(s):
N-Hydroxyaniline, N-Hydroxybenzenamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7NO
CAS Number:
Molecular Weight:
109.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
solid
mp
80-84 °C
storage temp.
−20°C
SMILES string
ONc1ccccc1
InChI
1S/C6H7NO/c8-7-6-4-2-1-3-5-6/h1-5,7-8H
InChI key
CKRZKMFTZCFYGB-UHFFFAOYSA-N
Application
N-Phenylhydroxylamine can be used as a starting material for the synthesis of:
- 2-alkylindoles by treating with aliphatic terminal alkynes using gold catalyst via sequential 3,3-rearrangements and cyclodehydrations.
- Isoxazolidines by reacting with aldehydes and α, β-unsaturated aldehydes via a three-component one-pot catalytic reaction.
- Tetrahydro-1,2-oxazines by treating with an aldehyde and cyclopropane via homo 3+2 dipolar cycloaddition reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Nohmi et al.
Mutation research, 136(3), 159-168 (1984-06-01)
Phenylhydroxylamine (PHA) and its derivatives such as monomethyl (2-Me, 3-Me, 4-Me) and dimethyl (2,3-diMe, 2,4-diMe, 2,5-diMe, 2,6-diMe, 3,4-diMe, 3,5-diMe) were tested for their mutagenicity and for their inducing ability to inactivate transforming DNA. All these compounds except PHA and 3,5-diMePHA
Copper-catalyzed amination of alkenes and ketones by phenylhydroxylamine.
Ho C-M and Lau T-C
New. J. Chem., 24(11), 859-863 (2000)
Au-catalyzed synthesis of 2-alkylindoles from N-arylhydroxylamines and terminal alkynes
Wang Y, et al.
Chemical Communications (Cambridge, England), 47(27), 7815-7817 (2011)
T P Bradshaw et al.
Free radical biology & medicine, 18(2), 279-285 (1995-02-01)
Previous studies have shown that incubation of rat red blood cells in vitro with phenylhydroxylamine (50-300 microM) induces rapid splenic sequestration of the red cells on reintroduction to isologous rats. EPR and the spin trapping agent, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), were utilized
C C Somerville et al.
Journal of bacteriology, 177(13), 3837-3842 (1995-07-01)
Pseudomonas pseudoalcaligenes JS45 grows on nitrobenzene as a sole source of carbon, nitrogen, and energy. The catabolic pathway involves reduction to hydroxylaminobenzene followed by rearrangement to o-amino-phenol and ring fission (S. F. Nishino and J. C. Spain, Appl. Environ. Microbiol.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service