All Photos(1)
About This Item
Empirical Formula (Hill Notation):
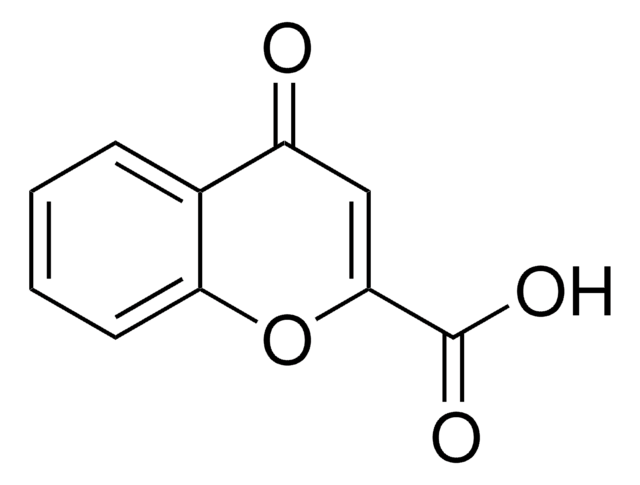
C10H6O4
CAS Number:
Molecular Weight:
190.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
202-205 °C (lit.)
functional group
carboxylic acid
ketone
SMILES string
OC(=O)C1=COc2ccccc2C1=O
InChI
1S/C10H6O4/c11-9-6-3-1-2-4-8(6)14-5-7(9)10(12)13/h1-5H,(H,12,13)
InChI key
PCIITXGDSHXTSN-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770)
General description
Chromone-3-carboxylic acid is a chromone derivative. Its potential as an antioxidant in charge transfer (CT) processes has been assessed by surface-enhanced Raman scattering (SERS) analysis of chromone 3-carboxylic acid adsorbed on silver colloids.
Application
Chromone-3-carboxylic acid may be used in the preparation of:
- chromane-2,4-diones
- chromone-3-carboxamides
- 5-(2-hydroxyphenyl)isoxazole
- chromone-2-carboxamides
- chromone-2-carboxamido-3-esters
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Conjugate addition of isocyanides to chromone 3-carboxylic acid: an efficient one-pot synthesis of chroman-4-one 2-carboxamides.
Neo AG, et al.
Organic & Biomolecular Chemistry, 10(17), 3406-3416 (2012)
Chromone-3-carboxylic acid as a potential electron scavenger: a surface-enhanced Raman scattering study.
Machado NFL, et al.
Physical Chemistry Chemical Physics, 13(3), 1012-1018 (2011)
F Cagide et al.
Chemical communications (Cambridge, England), 51(14), 2832-2835 (2015-01-13)
The discovery of potent and selective monoamine oxidase-B inhibitors for the management of neurodegenerative diseases such as Alzheimer's and Parkinson's diseases is still a challenging endeavor. Herein, we report the discovery of two new classes of potent and selective MAO-B
Synthesis of [1] benzopyrano [3,4-d] isoxazol-4-ones from 2-substituted chromone-3-carboxylic esters. A reinvestigation of the reaction of 3-acyl-4-hydroxycoumarins with hydroxylamine. Synthesis of 4-(2-hydroxybenzoyl) isoxazol-5-ones.
Chantegrel B, et al.
The Journal of Organic Chemistry, 49(23), 4419-4424 (1984)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service