All Photos(1)
About This Item
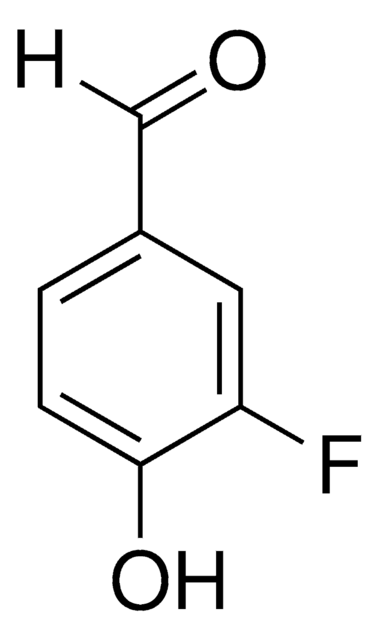
Linear Formula:
FC6H2(OH)(OCH3)CHO
CAS Number:
Molecular Weight:
170.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
114-118 °C (lit.)
functional group
aldehyde
fluoro
SMILES string
COc1cc(C=O)cc(F)c1O
InChI
1S/C8H7FO3/c1-12-7-3-5(4-10)2-6(9)8(7)11/h2-4,11H,1H3
InChI key
OOGOFUKAJDPHDJ-UHFFFAOYSA-N
General description
3-Fluoro-4-hydroxy-5-methoxybenzaldehyde is an aryl fluorinated building block.
Application
3-Fluoro-4-hydroxy-5-methoxybenzaldehyde may be used to synthesize 3-(3-fluoro-4-hydroxy-5-methoxyphenyl)-N-phenethylacrylamide and 4-[(4-hydroxy-3-fluoro-5-methoxy-benzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The new Schiff base 4-[(4-Hydroxy-3-fluoro-5-methoxy-benzylidene) amino]-1, 5-dimethyl-2-phenyl-1, 2-dihydro-pyrazol-3-one: Experimental, DFT calculational studies and in vitro antimicrobial activity.
Iskeleli NO, et al.
Spectrochimica Acta Part A: Molecular Spectroscopy, 139, 356-366 (2015)
John Yang et al.
Bioorganic & medicinal chemistry, 18(14), 5032-5038 (2010-07-06)
A series of catechol ring-fluorinated derivatives of caffeic acid phenethyl amide (CAPA) were synthesized and screened for cytoprotective activity against H2O2 induced oxidative stress in human umbilical vein endothelial cells (HUVEC). CAPA and three fluorinated analogs were found to be
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service