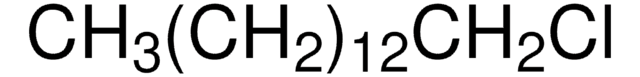292206
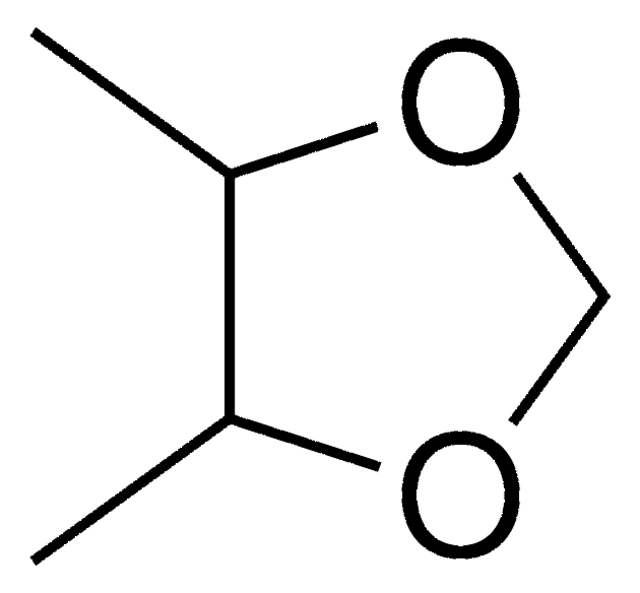
2-Methyl-1,3-dioxolane
97%
Synonym(s):
Acetaldehyde ethylene acetal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O2
CAS Number:
Molecular Weight:
88.11
Beilstein:
102520
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.398 (lit.)
bp
82-83 °C (lit.)
density
0.982 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
CC1OCCO1
InChI
1S/C4H8O2/c1-4-5-2-3-6-4/h4H,2-3H2,1H3
InChI key
HTWIZMNMTWYQRN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The kinetics and mechanism of the gas-phase thermal decomposition of 2-methyl-1,3-dioxolane has been studied in a static system. The infrared spectra of solid, liquid and gaseous 2-methyl-1,3-dioxolane has been studied. Low-temperature ozonation of 2-methyl-1,3-dioxolane in acetone-d6, methyl acetate and tert-butyl methyl ether has been reported.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
28.4 °F - closed cup
Flash Point(C)
-2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Watson et al.
Journal of autonomic pharmacology, 14(4), 283-293 (1994-08-01)
1. Muscarinic receptors mediating contraction of rabbit endothelium-denuded aorta have been characterized functionally, in vitro, using a range of antagonists (atropine, pirenzepine, methoctramine, himbacine, 4-diphenyl-acetoxy-N-methyl piperidine methiodide (4-DAMP) and para-fluoro-hexahydro-siladifenidol (p-F-HHSiD). 2. The non-selective muscarinic agonist, (+)cis-dioxolane, induced concentration-dependent contractions
G Blanchet et al.
Comptes rendus des seances de la Societe de biologie et de ses filiales, 178(5), 526-534 (1984-01-01)
The radioreceptor assay for acetylcholine (ACh) is based on the ability of the ACh to compete with the specific binding of [3H] cis-methyldioxolane to muscarinic receptors of rat cerebral cortex membranes. The technique described was used to measure ACh levels
Bozo Plesnicar et al.
Journal of the American Chemical Society, 124(38), 11260-11261 (2002-09-19)
Low-temperature ozonation (-78 degrees C) of 2-methyl-1,3-dioxolane (1a) in acetone-d6, methyl acetate, and tert-butyl methyl ether produced both the corresponding acetal hydrotrioxide (3a, ROOOH) and the hemiortho ester (2a, ROH) in molar ratio 1:5. Both intermediates were fully characterized by
E T Iwamoto et al.
The Journal of pharmacology and experimental therapeutics, 271(2), 601-608 (1994-11-01)
This study was designed to determine if the antinociception produced by intrathecally (i.t.) administered muscarinic agonists in male Sprague-Dawley rats is mediated by an L-arginine/nitric oxide/cyclic GMP cascade. Seven days after implantation of intrathecal catheters, antinociception was produced with graded
S Yamada et al.
Brain research, 410(2), 212-218 (1987-05-05)
To study the role of central cholinergic mechanisms in hypertension, we have determined nicotinic and muscarinic agonist binding sites in the brain regions of stroke-prone spontaneously hypertensive rats (SHRSP), using [3H]nicotine and [3H]cismethyldioxolane (CD). There was a significant decrease in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service