All Photos(1)
About This Item
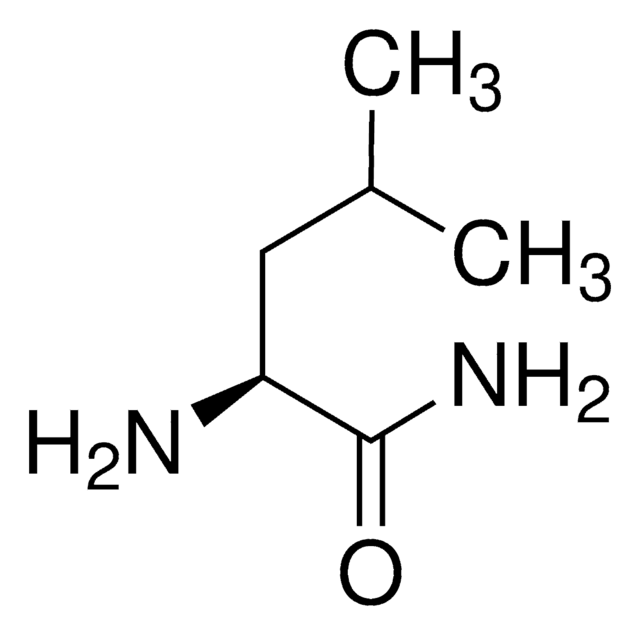
Linear Formula:
(CH3)2CHCH2CH(NH2)CONH2·HCl
CAS Number:
Molecular Weight:
166.65
Beilstein:
4237021
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]25/D +10°, c = 5 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
254-256 °C (lit.)
application(s)
peptide synthesis
SMILES string
Cl.CC(C)C[C@H](N)C(N)=O
InChI
1S/C6H14N2O.ClH/c1-4(2)3-5(7)6(8)9;/h4-5H,3,7H2,1-2H3,(H2,8,9);1H/t5-;/m0./s1
InChI key
VSPSRRBIXFUMOU-JEDNCBNOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[Marked dissociation of leucine aminopeptidase activities by the use of 2 different substrates--application of the methods to lymphatic diseases].
Y Hirasawa et al.
Nihon Ketsueki Gakkai zasshi : journal of Japan Haematological Society, 45(5), 907-912 (1982-09-01)
B Grinde et al.
Acta biologica et medica Germanica, 40(10-11), 1603-1612 (1981-01-01)
An amino acid mixture, specially designed to improve the protein balance in isolated hepatocytes, inhibited lysosomal (propylamine-sensitive) degradation of endogenous proteins by 80-90%. The amino acids had no effect on the degradation of the endocytosed protein asialofetuin, the conclusion being
Measurement of carprofen enantiomer concentrations in plasma and urine using L-leucinamide as the chiral coupling component.
H Spahn et al.
Journal of chromatography, 433, 331-338 (1988-12-09)
Formation of diastereomeric derivatives of 2-arylpropionic acids using L-leucinamide: lack of generality.
R Mehvar et al.
Journal of chromatography, 431(1), 228-230 (1988-09-23)
Formation of diastereomeric derivatives of 2-arylpropionic acids using L-leucinamide.
H Spahn
Journal of chromatography, 423, 334-339 (1987-12-25)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service