All Photos(2)
About This Item
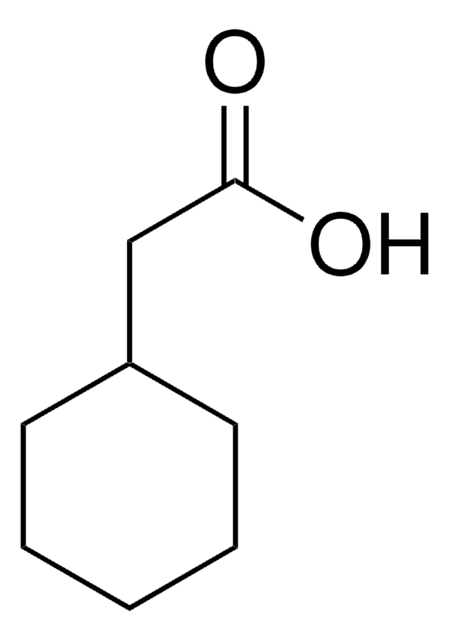
Linear Formula:
CH3C6H10CO2H
CAS Number:
Molecular Weight:
142.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
234 °C (lit.)
mp
36-39 °C (lit.)
functional group
carboxylic acid
SMILES string
CC1(CCCCC1)C(O)=O
InChI
1S/C8H14O2/c1-8(7(9)10)5-3-2-4-6-8/h2-6H2,1H3,(H,9,10)
InChI key
REHQLKUNRPCYEW-UHFFFAOYSA-N
Related Categories
General description
1-Methyl-1-cyclohexanecarboxylic acid is the structural analog of valproic acid and its pharmacokinetic action has been studied in female Sprague-Dawley rats.
Application
1-Methyl-1-cyclohexanecarboxylic acid was used as internal standard during the determination of valproic acid metabolites.
Biochem/physiol Actions
1-Methyl-1-cyclohexanecarboxylic acid is an anticonvulsant drug and causes maturation of murine neuroblastoma cells in vitro.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J L Vayssière et al.
FEBS letters, 173(1), 19-22 (1984-07-23)
Various mitochondrial inhibitors are tested in neuroblastoma cells. Their effects on the mit-proteins and some cytoskeletal proteins are compared to those of CCA, a differentiation inducer. This comparison favours the hypothesis that the primary effect of CCA induction is an
Effects of 1-methyl cyclohexane carboxylic acid (CCA) on cellular energetics in neuroblastoma cells.
B Croizat et al.
Biochemical and biophysical research communications, 103(3), 1044-1051 (1981-12-15)
J C Larcher et al.
Experimental cell research, 203(1), 72-79 (1992-11-01)
Adriamycin, an anticancer agent acting on topoisomerase II, promotes the arrest of cell division and neurite extension in a "neurite-minus" murine neuroblastoma cell line, N1A-103. This morphological differentiation is accompanied by a blockade in the S phase of the cell
P Benoit et al.
Neuropharmacology, 21(12), 1239-1244 (1982-12-01)
The effect of an anticonvulsant compound (Simiand, Ferrandes, Lacolle and Eymard, 1979), 1-methyl cyclohexane carboxylic acid (CCA), upon the electrical activity of Purkinje cells (PCs) was studied in the cerebellar cortex of the rat in vivo. Cyclohexane carboxylic acid (200-400
J C Larcher et al.
Oncogene, 6(4), 633-638 (1991-04-01)
Using clones N1E-115 and N1A-103 from mouse neuroblastoma C1300, a comparative analysis of c- and N-myc gene expression was undertaken both in proliferating cells and in cultures exposed to conditions which induce differentiation. Under the latter conditions, while N1E-115 cells
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service