All Photos(1)
About This Item
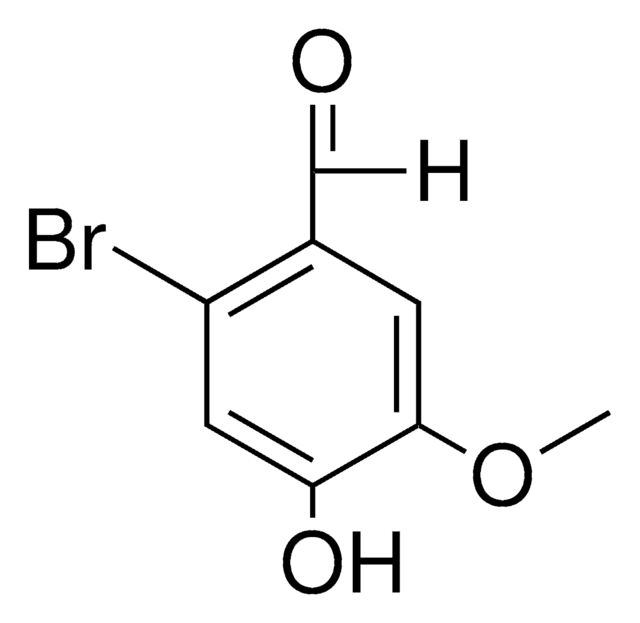
Linear Formula:
BrC6H2-4-(OH)-3-(OCH3)CHO
CAS Number:
Molecular Weight:
231.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
164-166 °C (lit.)
functional group
aldehyde
bromo
SMILES string
COc1cc(C=O)cc(Br)c1O
InChI
1S/C8H7BrO3/c1-12-7-3-5(4-10)2-6(9)8(7)11/h2-4,11H,1H3
InChI key
KLSHZDPXXKAHIJ-UHFFFAOYSA-N
Application
5-Bromovanillin was used to enrich the metabolically stable anaerobic cultures to study dechlorination of chlorocatechols. It was also used to prepare 2, 5-dihydroxy-4-methoxy-6-bromobenzaldehyde and 5-bromovanillate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A S Allard et al.
Applied and environmental microbiology, 57(1), 77-84 (1991-01-01)
Metabolically stable anaerobic cultures obtained by enrichment with 5-bromovanillin, 5-chlorovanillin, catechin, and phloroglucinol were used to study dechlorination of chlorocatechols. A high degree of specificity in dechlorination was observed, and some chlorocatechols were appreciably more resistant to dechlorination than others:
P J Kersten et al.
Journal of bacteriology, 162(2), 693-697 (1985-05-01)
Four strains of gram-negative bacteria capable of growing at the expense of 5-chlorovanillate were isolated from soil, and the metabolism of one strain was studied in particular detail. In the presence of alpha, alpha'-bipyridyl, a suspension of 5-chlorovanillate-grown cells accumulated
G Martin et al.
European journal of biochemistry, 261(2), 533-539 (1999-04-24)
The Burkholderia cepacia AC1100 strain, known to degrade the herbicide, 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T), is able to metabolize 4-hydroxyarylaldehyde, not only into the corresponding acid, but also into a new hydroquinone, 2,5-dihydroxyarylaldehyde. When incubated with resting AC1100 cells or cell-free extracts
Roland Tolulope Loto
Scientific reports, 7(1), 17555-17555 (2017-12-16)
The synergistic properties of the combined admixture of benzenecarbonitrile and 5-bromovanillin (BNV) on the corrosion resistance of 1018 carbon steel in 1 M HCl was analysed with potentiodynamic polarization technique, weight loss method, micro-analytical studies and ATF-FTIR spectroscopy. Results obtained show
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service