D7815
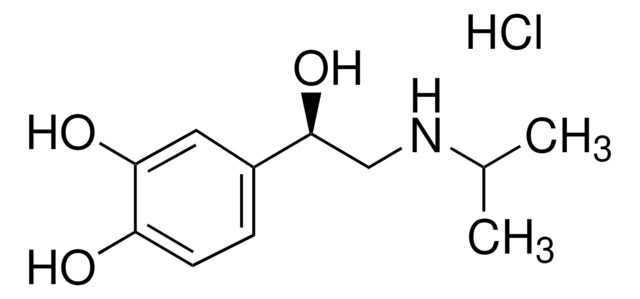
R(−)-Denopamine
≥98% (HPLC), powder
Synonym(s):
(−)-α-(3,4-Dimethoxyphenethylaminomethyl)-4-hydroxybenzylalcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H23NO4
CAS Number:
Molecular Weight:
317.38
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white
solubility
DMSO: 10 mg/mL, clear
H2O: insoluble
storage temp.
2-8°C
SMILES string
COc1ccc(CCNC[C@H](O)c2ccc(O)cc2)cc1OC
InChI
1S/C18H23NO4/c1-22-17-8-3-13(11-18(17)23-2)9-10-19-12-16(21)14-4-6-15(20)7-5-14/h3-8,11,16,19-21H,9-10,12H2,1-2H3/t16-/m0/s1
InChI key
VHSBBVZJABQOSG-INIZCTEOSA-N
Gene Information
human ... ADRB1(153)
Application
R(-)-Denopamine has been used as an β-adrenergic agonist to study its effects on contractility in isolated lymphatic vessels (LVs).
Biochem/physiol Actions
Denopamine is a cardiotonic drug.
β1-adrenoceptor agonist.
Features and Benefits
This compound is featured on the β-Adrenoceptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Caution
Light sensitive.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Weak arrhythmogenic property of the new cardiotonic agent denopamine in dogs: comparison with catecholamines
NARITA H, et al.
Japanese Journal of Pharmacology, 41(3), 335-344 (1986)
M Hirayama et al.
Rinsho shinkeigaku = Clinical neurology, 40(8), 787-790 (2001-02-24)
In 1993, we reported the pathophysiology of postprandial hypotension (PPH) in patients with sympathetic dysfunction: a fall of BP resulted from both excess systemic vasodilation and lack of compensatory increase of cardiac output and vascular resistance in the leg arteries.
Gao Shang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(27), 7780-7784 (2007-06-27)
Two beta-receptor agonists (-)-denopamine and (-)-arbutamine were prepared in good yields and enantioselectivities by asymmetric hydrogenation of unprotected amino ketones for the first time by using Rh catalysts bearing electron-donating phosphine ligands. A series of alpha-primary and secondary amino ketones
Ruduwaan Salie et al.
Cardiovascular drugs and therapy, 25(1), 31-46 (2011-01-13)
To determine the mechanism whereby transient stimulation of the β-adrenergic receptor subtypes (β-AR) elicit cardioprotection against subsequent ischaemia. Isolated rat hearts were subjected to 35 min regional ischaemia (RI) and reperfusion and infarct size (IS) determined. Hearts were preconditioned with
Barry M Trost et al.
Organic letters, 4(16), 2621-2623 (2002-08-03)
[reaction: see text] Syntheses of variously modified ligands for the dinuclear zinc catalysts for the asymmetric aldol and nitroaldol (Henry) reactions are reported. Catalytic enantioselective nitroaldol reactions promoted by these modified ligands led to efficient syntheses of the beta-receptor agonists
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service