About This Item
Recommended Products
grade
analytical standard
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
application(s)
agriculture
environmental
format
neat
storage temp.
2-8°C
SMILES string
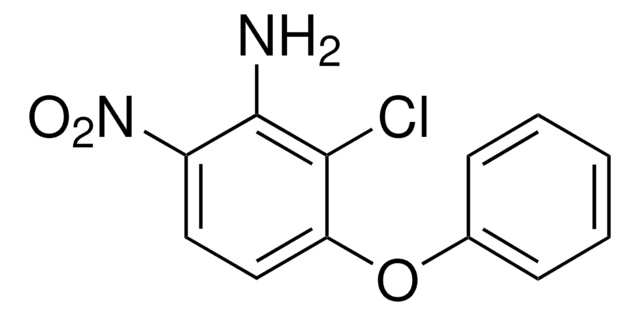
CC1(C)CON(Cc2ccccc2Cl)C1=O
InChI
1S/C12H14ClNO2/c1-12(2)8-16-14(11(12)15)7-9-5-3-4-6-10(9)13/h3-6H,7-8H2,1-2H3
InChI key
KIEDNEWSYUYDSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Ecological risk assessment of Clomazone in Northeast China: A study analyzed the presence and distribution of herbicide residues, including Clomazone, in soils across the Mollisols region, assessing their ecological risks. This research highlights the environmental impact of such herbicides and informs crop safety protocols and regulatory standards (Li et al., 2024).
- Characterization of Clomazone sorption in agricultural soils: This research characterized the sorption behavior of Clomazone in various agricultural soils using different kinetic models, contributing to a better understanding of its environmental interactions and aiding in the improvement of its application strategies for effective weed control (Ðurović-Pejčev et al., 2023).
- Bioassay assessment of water toxicity including Clomazone: A study utilized bioassays to assess the toxicity of water samples from Brazilian rural areas, implicating several contaminants including Clomazone. The findings are crucial for evaluating the safety of drinking water and the implications of herbicide runoff in agricultural settings (Brucker et al., 2021).
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
314.6 °F
Flash Point(C)
157 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service