901097
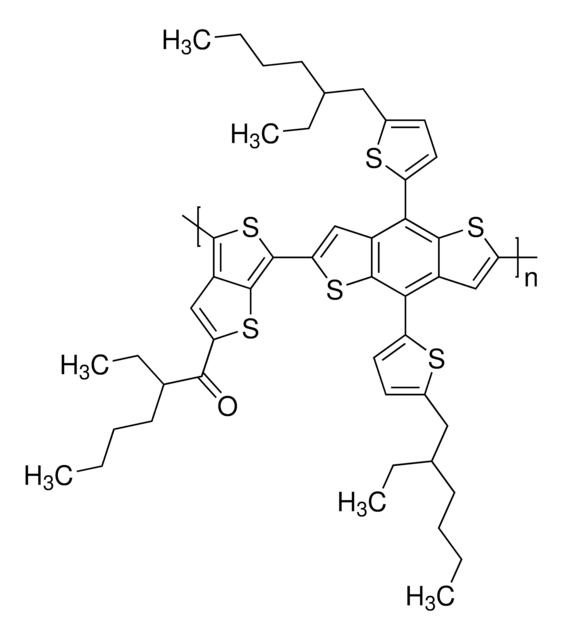
PDBT-T1
Synonym(s):
Poly[[5,10-bis(5-octyl-2-thienyl)dithieno[2,3-d:2′,3′-d′]benzo[1,2-b:4,5-b′]dithiophene-2,7-diyl]-2,5-thiophenediyl[5,7-bis(2-ethylhexyl)-4,8-dioxo-4H,8H-benzo[1,2-c:4,5-c′]dithiophene-1,3-diyl]-2,5-thiophenediyl]
About This Item
Recommended Products
description
Band gap: 1.93 eV
Quality Level
form
solid
mol wt
Mw 20,000-50,000 (GPC, PS standard)
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>200 °C
solubility
soluble (hot o-dichlorobenzene)
Orbital energy
HOMO -5.36 eV
LUMO -3.43 eV
greener alternative category
, Enabling
General description
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Novel biosensing devices, like bio-FET sensors, offer label-free analysis by detecting ionic and biomolecular charges, crucial for diagnostics and biological research.
Novel biosensing devices, like bio-FET sensors, offer label-free analysis by detecting ionic and biomolecular charges, crucial for diagnostics and biological research.
Novel biosensing devices, like bio-FET sensors, offer label-free analysis by detecting ionic and biomolecular charges, crucial for diagnostics and biological research.
Novel biosensing devices, like bio-FET sensors, offer label-free analysis by detecting ionic and biomolecular charges, crucial for diagnostics and biological research.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service