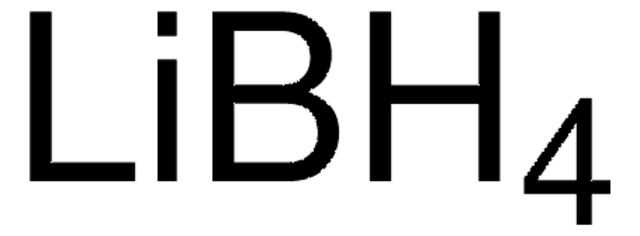702714
Lithium borohydride solution
0.5 M in diethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
LiBH4
CAS Number:
Molecular Weight:
21.78
MDL number:
UNSPSC Code:
26111700
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
reaction suitability
reagent type: reductant
concentration
0.5 M in diethyl ether
density
0.719 g/mL at 25 °C
SMILES string
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI key
UUKMSDRCXNLYOO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Lithium borohydride is a commonly used reducing agent. It can also be used a reactant for:
- Preparation of gallium, indium, rhenium and zinc tris(mercaptoimidazolyl)hydroborato complexes.
- Mechano-chemical metathesis reactions.
- Noncatalytic hydrolysis for hydrogen generation.
- Growth of large gold monolayer protected-clusters.
- Anion substitution reactions.
- Dehydrogenation reactions.
Reactant for:
- Preparation of gallium, indium, rhenium and zinc tris(mercaptoimidazolyl)hydroborato complexes
- Mechano-chemical metathesis reactions
- Noncatalytic hydrolysis for hydrogen generation
- Growth of large gold monolayer protected-clusters
- Anion substitution reactions
- Dehydrogenation reactions
Other Notes
Material may form precipitate on standing, which does not affect its use.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
-40.0 °F
Flash Point(C)
-40 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Controlled growth and catalytic activity of gold monolayer protected clusters in presence of borohydride salts.
Dasog M, et al.
Chemical Communications (Cambridge, England), 47(30), 8569-8571 (2011)
Effect of hydrogen back pressure on dehydrogenation behavior of LiBH4-based reactive hydride composites.
Shim J H, et al.
The Journal of Physical Chemistry Letters, 1(1), 59-63 (2009)
Lithium Borohydride.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2009)
Synthesis, crystal structure, and thermal properties of the first mixed-metal and anion-substituted rare earth borohydride LiCe (BH4) 3Cl.
Frommen c, et al.
The Journal of Physical Chemistry, 115(47), 23591-23602 (2011)
Mechano-chemical reactions in LiBH4+ VCln (n= 2 and 3) mixtures.
Llamas-Jansa I, et al.
J. Alloy Compounds, 509, S684-S687 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service