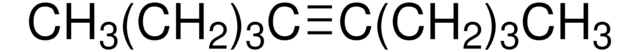680834
N-(2,2,2-Trichloroethoxysulfonyl)urea
96%
Synonym(s):
Aminocarbonylsulfamic acid, 3,3,3-trichloroethoxy ester, Tces-Urea
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CONHSO2OCH2CCl3
CAS Number:
Molecular Weight:
271.51
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
161-165 °C
SMILES string
NC(=O)NS(=O)(=O)OCC(Cl)(Cl)Cl
InChI
1S/C3H5Cl3N2O4S/c4-3(5,6)1-12-13(10,11)8-2(7)9/h1H2,(H3,7,8,9)
InChI key
QYUJBOJJEGIBCJ-UHFFFAOYSA-N
Application
- Reactant for oxidative C-H amination reactions
Reagent for Rh-catalyzed urea formation.
Reagent for cyclic urea synthesis.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mihyong Kim et al.
Organic letters, 8(6), 1073-1076 (2006-03-10)
[reaction: see text] Oxidative C-H amination of N-trichloroethoxysulfonyl-protected ureas and guanidines is demonstrated to proceed in high yield for tertiary and benzylic-derived substrates. The success of these reactions is predicated on the choice of the electron-withdrawn 2,2,2-trichloroethoxysulfonyl (Tces) protecting group
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Bis[rhodium(α,α,α′,α′-tetramethyl-1,3-benzenedipropionic acid)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)