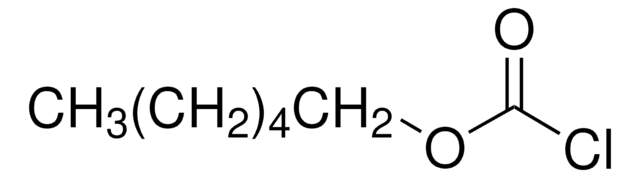460931
Neopentyl chloroformate
97%
Synonym(s):
2,2-Dimethylpropyl chloroformate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CCH2OCOCl
CAS Number:
Molecular Weight:
150.60
Beilstein:
506655
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
−0.65 psi ( 20 °C)
Assay
97%
refractive index
n20/D 1.410 (lit.)
bp
55 °C/36 mmHg (lit.)
density
1.003 g/mL at 25 °C (lit.)
functional group
chloro
storage temp.
2-8°C
SMILES string
CC(C)(C)COC(Cl)=O
InChI
1S/C6H11ClO2/c1-6(2,3)4-9-5(7)8/h4H2,1-3H3
InChI key
JUUBFHLPTCPVBO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Neopentyl chloroformate, a branched primary alkyl chloroformate, is a useful protecting agent during peptide synthesis.
Application
Neopentyl chloroformate was used in the preparation of mixture of 11,12-dimethyltetradecanoic acid and 11-ethyl-12-methyltridecanoic acid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
89.6 °F - closed cup
Flash Point(C)
32 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ursula Biermann et al.
Journal of the American Chemical Society, 126(33), 10319-10330 (2004-08-19)
A general method for the hydro-alkyl addition to the nonactivated C=C double bond of alkenes using alkyl chloroformates (primary, secondary), 12, and di-tert-butylpyrocarbonate, 52, mediated by ethylaluminum sesquichloride (Et(3)Al(2)Cl(3)) has been developed. Reaction of 12 and 52, respectively, with Et(3)Al(2)Cl(3)
Malcolm J D'Souza et al.
International journal of molecular sciences, 12(2), 1161-1174 (2011-05-05)
The specific rates of solvolysis of neopentyl chloroformate (1) have been determined in 21 pure and binary solvents at 45.0 °C. In most solvents the values are essentially identical to those for ethyl and n-propyl chloroformates. However, in aqueous-1,1,1,3,3,3-hexafluoro-2-propanol mixtures
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service