368113
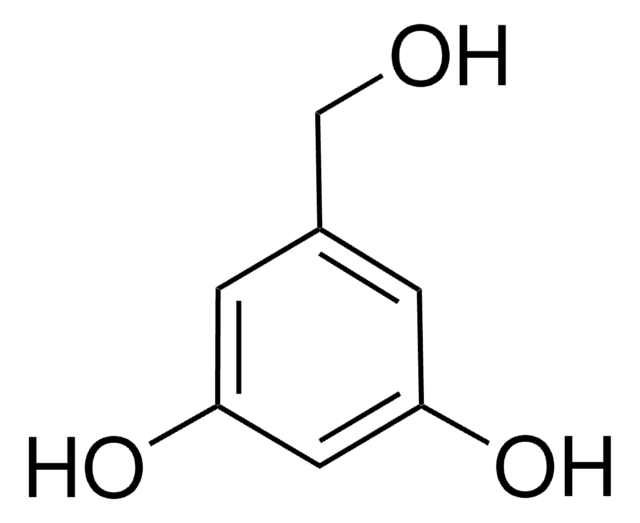
3,5-Dihydroxybenzaldehyde
98%
Synonym(s):
α-Resorcylaldehyde (6CI)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(HO)2C6H3CHO
CAS Number:
Molecular Weight:
138.12
Beilstein:
1930147
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
153-158 °C (lit.)
SMILES string
[H]C(=O)c1cc(O)cc(O)c1
InChI
1S/C7H6O3/c8-4-5-1-6(9)3-7(10)2-5/h1-4,9-10H
InChI key
HAQLHRYUDBKTJG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Condensation of isopropyl ethers of p-hydroxyphenyl acetic acid and 3,5-dihydroxybenzaldehyde has been reported.
Application
3,5-Dihydroxybenzaldehyde is suitable for use in the synthesis of 3,5-dihydroxyphenylglycine (3,5-DHPG). It may be used in the synthesis of:
- fulgide7
- 3,5-bis(undeca-4,6-diynyloxy)benzaldehyde
- 3,5-didodecyloxybenzaldehyde
- 1′-methyl-1′,5′-dihydro-2′-(3,5-bis(undeca-4,6-diynyloxy)phenyl)-1H-pyrrolo[3′,4′:1,9](C60-Ih)[5,6]fullerene (F2D)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and photochromism of E, E-3, 4-(3, 5-dimethoxybenzylidene) succinic anhydride and its infra red active 2-dicyanomethylene derivative.
Asiri AM.
Journal of Photochemistry and Photobiology A: Chemistry, 159(1), 1-5 (2003)
A re-investigation of resveratrol synthesis by Perkins reaction. Application to the synthesis of aryl cinnamic acids.
Solladie G, et al.
Tetrahedron, 59(18), 3315-3321 (2003)
Enzymatic resolution and pharmacological activity of the enantiomers of 3, 5-dihydroxyphenylglycine, a metabotropic glutamate receptor agonist.
Richard BS, et al.
Bioorganic & Medicinal Chemistry Letters, 5(3), 223-228 (1995)
Denis E Markov et al.
The journal of physical chemistry. A, 109(24), 5266-5274 (2006-07-15)
Exciton diffusion and photoluminescence quenching in conjugated polymer/fullerene heterostructures are studied by time-resolved photoluminescence. It is observed that heterostructures consisting of a spin-coated poly(p-phenylene vinylene) (PPV)-based derivative and evaporated C60 are ill-defined because of diffusion of C60 into the polymer
Ivan K Ilic et al.
ChemSusChem, 13(7), 1856-1863 (2020-02-07)
Although several recent publications describe cathodes for electrochemical energy storage materials made from regrown biomass in aqueous electrolytes, their transfer to lithium-organic batteries is challenging. To gain a deeper understanding, we investigate the influences on charge storage in model systems
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service