All Photos(1)
About This Item
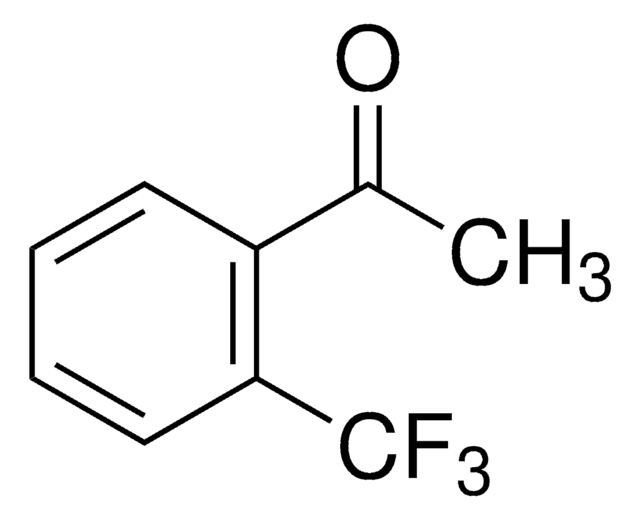
Linear Formula:
(CF3)2C6H3COCH3
CAS Number:
Molecular Weight:
256.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.4221 (lit.)
density
1.422 g/mL at 25 °C (lit.)
functional group
fluoro
ketone
SMILES string
CC(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F
InChI
1S/C10H6F6O/c1-5(17)6-2-7(9(11,12)13)4-8(3-6)10(14,15)16/h2-4H,1H3
InChI key
MCYCSIKSZLARBD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Kneubühler et al.
Journal of medicinal chemistry, 38(19), 3874-3883 (1995-09-15)
A large series (66 compounds) of indeno[1,2-c]pyridazin-5-ones (IPs) were synthesized and tested on their monoamine oxidase-A (MAO-A) and MAO-B inhibitory activity. All of the tested compounds acted preferentially on MAO-B displaying weak (nonmeasurable IC50 values) to high (submicromolar IC50 values)
Pu Wang et al.
Applied microbiology and biotechnology, 90(6), 1897-1904 (2011-05-27)
A novel bacterial strain HS0904 was isolated from a soil sample using 3,5-bis(trifluoromethyl) acetophenone as the sole carbon source. This bacterial isolate can asymmetrically reduce 3,5-bis(trifluoromethyl) acetophenone to (1R)-[3,5-bis(trifluoromethyl)phenyl] ethanol with high enantiometric excess (ee) value. Based on its morphological
Rhodium-catalyzed heterogeneous enantioselective hydrogenation of 3, 5-di-(trifluoromethyl)-acetophenone.
Hess R, et al.
J. Mol. Catal. A: Chem., 212(1), 205-209 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service