All Photos(1)
About This Item
Empirical Formula (Hill Notation):
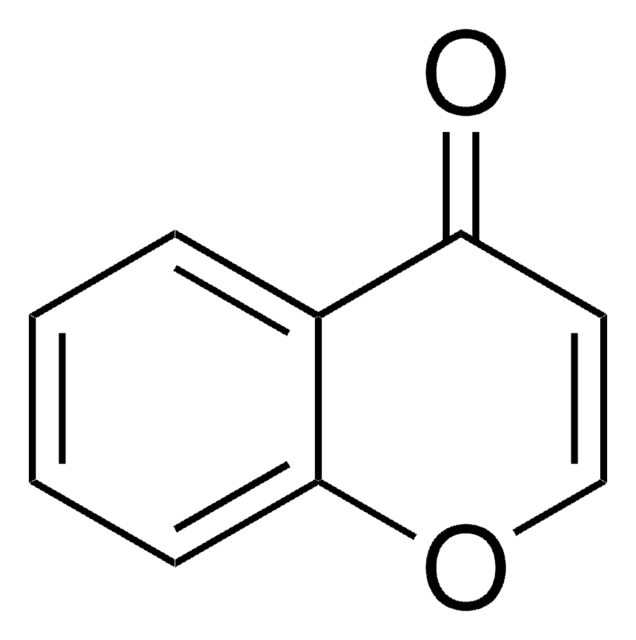
C10H10O2
CAS Number:
Molecular Weight:
162.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
141-143 °C/13.5 mmHg (lit.)
mp
31-35 °C (lit.)
density
1.071 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
Cc1ccc2OCCC(=O)c2c1
InChI
1S/C10H10O2/c1-7-2-3-10-8(6-7)9(11)4-5-12-10/h2-3,6H,4-5H2,1H3
InChI key
RJHXEPLSJAVTFW-UHFFFAOYSA-N
Application
The product has been used as a substrate for carbonyl reductase from Sporobolomyces salmonicolor, wherein it was reduced to the corresponding (R)-chiral alcohol. It has also been used as a substrate for the enantioselective reduction by 3 biocatalysts, namely, Didymosphaeria igniaria, Coryneum betulinum and Chaetomium sp.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enantioselective reduction of 4-chromanone and its derivatives by selected filamentous fungi.
Janeczko T, et al.
Journal of Molecular Catalysis. B, Enzymatic, 97, 278-282 (2013)
Dunming Zhu et al.
Organic & biomolecular chemistry, 4(14), 2690-2695 (2006-07-11)
In our effort to search for effective carbonyl reductases, the activity and enantioselectivity of a carbonyl reductase from Sporobolomyces salmonicolor have been evaluated toward the reduction of a variety of ketones. This carbonyl reductase (SSCR) reduces a broad spectrum of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service