All Photos(2)
About This Item
Empirical Formula (Hill Notation):
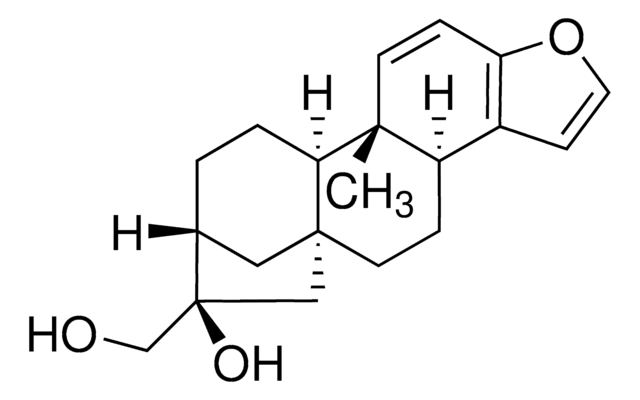
C22H30O4
CAS Number:
Molecular Weight:
358.47
MDL number:
UNSPSC Code:
12352205
PubChem Substance ID:
NACRES:
NA.79
Recommended Products
Assay
≥98%
form
powder
technique(s)
HPLC: suitable
color
white to off-white
storage temp.
2-8°C
SMILES string
CC(=O)OCC1(O)CC23CCC4c5ccoc5CCC4(C)C2CCC1C3
InChI
1S/C22H30O4/c1-14(23)26-13-22(24)12-21-9-5-17-16-7-10-25-18(16)6-8-20(17,2)19(21)4-3-15(22)11-21/h7,10,15,17,19,24H,3-6,8-9,11-13H2,1-2H3
InChI key
PTGGVIKFNQSFBY-UHFFFAOYSA-N
Application
Cafestol acetate has been used to determine the effects of coffee on heat shock response (HSR).
Biochem/physiol Actions
Cafestol is a coffee-specific diterpene. It has anti-carcinogenic and anti-inflammatory activities. Cafestol stimulates apoptosis in cells associated with colorectal and renal cancer (Caki cells). It is used to study mechanisms of anti-oxidation related to hydrogen peroxide induced oxidative stress and DNA damage. Cafestol increases the level of glutathione (GSH), by stimulating γ-glutamylcysteine synthetase. It acts as a chemopreventive agent by inducing cytochrome P-450.
Other Notes
Furan-containing diterpene from green coffee beans.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Wolfgang W Huber et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 46(4), 1230-1238 (2007-11-07)
Coffee drinking appears to reduce cancer risk in liver and colon. Such chemoprevention may be caused by the diterpenes kahweol and cafestol (K/C) contained in unfiltered beverage. In animals, K/C treatment inhibited the mutagenicity/tumorigenicity of several carcinogens, likely explicable by
Use of silver nitrate impregnated silica cartridges in the separation of kahweol and cafestol esters by preparative liquid chromatography.
L K Lam et al.
Journal of chromatography, 328, 422-424 (1985-06-28)
Agnieszka Potęga et al.
Journal of pharmaceutical analysis, 10(4), 376-384 (2020-09-15)
5-Dimethylaminopropylamino-8-hydroxytriazoloacridinone (C-1305) is a promising antitumor compound developed in our laboratory. A better understanding of its metabolic transformations is still needed to explain the multidirectional mechanism of pharmacological action of triazoloacridinone derivatives at all. Thus, the aim of the current
Cafestol, a diterpene molecule found in coffee, induces leukemia cell death
Lima C, et al.
Biomedicine and Pharmacotherapy, 92, 1045-1054 (2017)
Kyung Jin Lee et al.
Toxicology letters, 173(2), 80-87 (2007-08-11)
There is an increasing evidence that oxidative stress is implicated in the processes of inflammation and carcinogenesis. It has been shown that kahweol and cafestol, coffee-specific diterpenes, exhibit chemoprotective effects. This study investigated the effects of kahweol and cafestol, coffee-specific
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service