850993
2-Aminoisobutyric acid
98%, for peptide synthesis
Synonym(s):
α-Aminoisobutyric acid, 2-Methylalanine, Aib
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
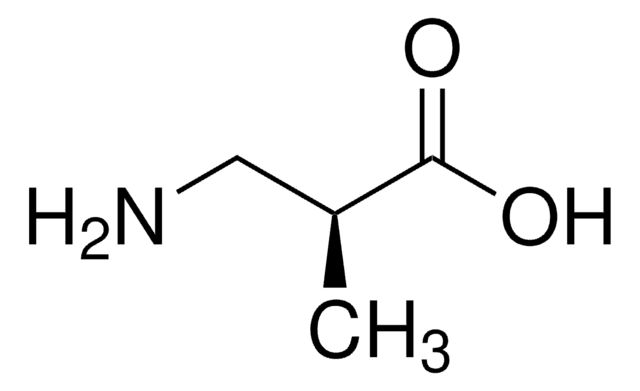
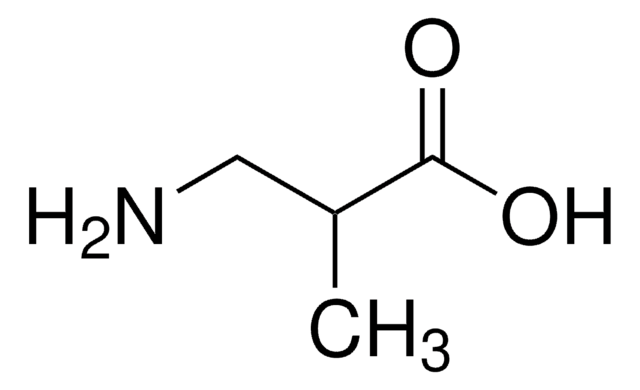
(CH3)2C(NH2)COOH
CAS Number:
Molecular Weight:
103.12
Beilstein:
506496
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Product Name
2-Aminoisobutyric acid, 98%
Assay
98%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
≥300 °C
application(s)
peptide synthesis
SMILES string
CC(C)(N)C(O)=O
InChI
1S/C4H9NO2/c1-4(2,5)3(6)7/h5H2,1-2H3,(H,6,7)
InChI key
FUOOLUPWFVMBKG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Aminoisobutyric acid also known as α-aminobutyric acid, is an amino acid used in solution-phase peptide synthesis. It is a desirable building block for peptides because of its strong tendency to cause the peptide to form a helical shape.
Application
2-Aminoisobutyric acid can be used to synthesize self-assembled polypeptide nanoparticles. Incorporation of this compound into the peptide chain can prevent undesired reactions since it is di-α-substituted, and inert to C−H abstraction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photochemical methods for peptide macrocyclisation
Chemistry?A European Journal , 27, 69-88 (2021)
Amphiphilic polypeptides with prolonged enzymatic stability for the preparation of self-assembled nanobiomaterials
Royal Society of Chemistry Advances, 8, 34603-34613 (2018)
Evgeniy Salnikov et al.
Journal of biomolecular NMR, 45(4), 373-387 (2009-10-14)
In protein NMR spectroscopy the chemical shift provides important information for the assignment of residues and a first structural evaluation of dihedral angles. Furthermore, angular restraints are obtained from oriented samples by solution and solid-state NMR spectroscopic approaches. Whereas the
Lucia Becucci et al.
Journal of the American Chemical Society, 132(17), 6194-6204 (2010-04-16)
Four oligopeptides consisting of a sequence of alpha-aminoisobutyric acid (Aib) residues, thiolated at either the N- or C-terminus by means of a -(CH(2))(2)-SH anchor, were self-assembled on mercury, which is a substrate known to impart a high fluidity to self-assembled
Marta De Zotti et al.
Amino acids, 43(4), 1761-1777 (2012-04-10)
The lipopeptaibol trichogin GA IV is a natural, non-ribosomally synthesized, antimicrobial peptide remarkably resistant to the action of hydrolytic enzymes. This feature may be connected to the multiple presence in its sequence of the non-coded residue α-aminoisobutyric acid (Aib), which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service