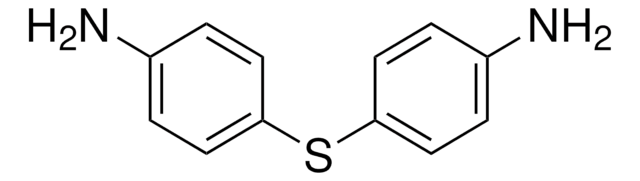
369462
4-Aminophenyl disulfide
98%
Synonym(s):
4,4′-Dithiodianiline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(H2NC6H4S-)2
CAS Number:
Molecular Weight:
248.37
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
powder
mp
77-78 °C (lit.)
SMILES string
Nc1ccc(SSc2ccc(N)cc2)cc1
InChI
1S/C12H12N2S2/c13-9-1-5-11(6-2-9)15-16-12-7-3-10(14)4-8-12/h1-8H,13-14H2
InChI key
MERLDGDYUMSLAY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Aminophenyl disulfide is primarily used as a reagent in chemical synthesis. It has thiol-disulfide interchange properties, making it useful in redox reactions and the synthesis of polymers. It can participate in thiol-disulfide exchange reactions, facilitating the formation of covalent bonds with other thiols or disulfides. 4-Aminophenyl disulfide is often employed as a crosslinking agent, particularly in polymerization reactions, where it can enhance the mechanical and thermal properties of polymers. It can also act as a dopant or charge-transfer agent in conductive polymers, improving their electrical conductivity.
Application
4-Aminophenyl disulfide can be used as:
Bioconjugation: The amine group can react with carboxylic acids or their derivatives, making it useful for conjugating biomolecules like proteins and peptides. This is particularly valuable in developing targeted drug delivery systems where the drug is directly linked to a molecule that can home in on cancer cells or other disease sites.
Diagnostic Agents: It can be used to modify surfaces of diagnostic devices, such as biosensors. They can help in the immobilization of biomolecules on sensor surfaces, improving the sensitivity and specificity of detection.
- A crosslinker in the synthesis of self-healing poly(urea-urethane) elastomers because of its quantitative healing efficiency. These self-healing materials find applications in various fields such as drug delivery, wound healing, and tissue engineering, electronics, aerospace, and coatings.
- A hardener in the formulation of re-processable, repairable, and recyclable epoxy networks due to the presence of dynamic disulfide bonds, which enable the epoxy networks to undergo chemical recycling. These epoxy networks are widely used in various applications which include, composite materials, coatings, adhesives, and fiber-reinforced thermoset composites.
- A crosslinking agent for the development of epoxy vitrimers and carbon fiber composites with enhanced mechanical properties, multi-shape memory capabilities for the potential applications in high-performance materials.
Bioconjugation: The amine group can react with carboxylic acids or their derivatives, making it useful for conjugating biomolecules like proteins and peptides. This is particularly valuable in developing targeted drug delivery systems where the drug is directly linked to a molecule that can home in on cancer cells or other disease sites.
Diagnostic Agents: It can be used to modify surfaces of diagnostic devices, such as biosensors. They can help in the immobilization of biomolecules on sensor surfaces, improving the sensitivity and specificity of detection.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Domenget et al.
Biochimica et biophysica acta, 830(1), 71-79 (1985-07-18)
The effects of four thiol reagents on the kinetics of polymerization of hemoglobin S have been studied in high phosphate buffer (1.8 M), in the presence (3 mM) or absence of sodium dithionite, depending on the reduction of mixed disulfides
Martin L Henriksen et al.
ChemSusChem, 10(14), 2936-2944 (2017-05-31)
Epoxy-based thermosets are one of the most popular matrix materials in many industries, and significant environmental benefits can be obtained by developing a recyclable variant of this widely utilized material. Incorporation of a bio-based disulfide additive within a commercial epoxy
Y Maede et al.
Blood, 73(1), 312-317 (1989-01-01)
It has been shown that certain dogs have erythrocytes characterized by an inherited high concentration of reduced glutathione (GSH), five to seven times the normal level (high-GSH RBCs). We examined whether increased GSH in dog erythrocytes leads to increased protection
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service