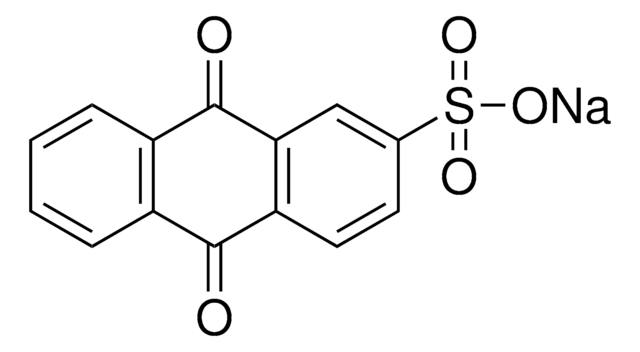
252727
Anthraquinone-2-carboxylic acid
98%
Synonym(s):
9,10-Dihydro-9,10-dioxo-2-anthracenecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H8O4
CAS Number:
Molecular Weight:
252.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
287-289 °C (lit.)
SMILES string
OC(=O)c1ccc2C(=O)c3ccccc3C(=O)c2c1
InChI
1S/C15H8O4/c16-13-9-3-1-2-4-10(9)14(17)12-7-8(15(18)19)5-6-11(12)13/h1-7H,(H,18,19)
InChI key
ASDLSKCKYGVMAI-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Kino et al.
Chemistry & biology, 8(4), 369-378 (2001-04-28)
The genome is constantly assaulted by oxidation reactions which are likely to be associated with oxygen metabolism, and oxidative lesions are generated by many types of oxidants. Such genotoxin-induced alterations in the genomic message have been implicated in aging and
Morihide Higo et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 24(3), 313-320 (2008-03-12)
The adsorption state and morphology of anthraquinone-2-carboxylic acid (AQ-2-COOH) deposited from acetone solutions (0.02 - 1.00 mg ml(-1)) onto atomically-smooth native oxide surfaces of Al(111) films were investigated by infrared reflection absorption spectroscopy, X-ray photoelectron spectroscopy, and atomic force microscopy.
Jie Sun et al.
Analytical chemistry, 90(11), 6660-6665 (2018-05-15)
To detect the redox state evolution during wound healing process, a redox-sensitive surface-enhanced Raman scattering (SERS) probe was constructed by attaching anthraquinone as a redox-sensitive molecule onto gold nanoshells, and the redox-sensitive SERS probes were loaded on one surface of
A Bielawska et al.
Polish journal of pharmacology, 53(3), 283-287 (2002-01-12)
A series of proline analogues of anthraquinone-2-carboxylic acid (1-3) were synthesized and evaluated for cytotoxic activity in the cultured breast cancer MCF-7 cells. The concentrations of 1, 2 and 3 needed to inhibit [3H]thymidine incorporation into DNA by 50% (IC50)
A Bielawska et al.
Roczniki Akademii Medycznej w Bialymstoku (1995), 43, 201-209 (1999-02-11)
The feasibility to targeting prolidase as an antineoplastic prodrug--converting enzyme has been examined. The synthesis of proline analogue of anthraquinone-2-carboxylic acid (potential antineoplastic agent) conjugated through imido-bond (potential target for prolidase action) has been performed. The product was found to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service