All Photos(1)
About This Item
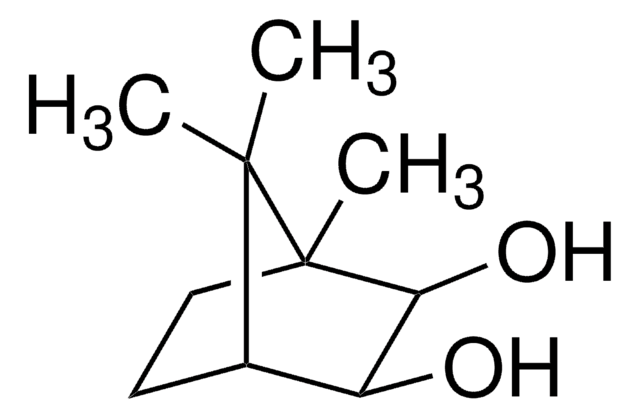
Empirical Formula (Hill Notation):
C10H16O
CAS Number:
Molecular Weight:
152.23
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
102-103 °C/50 mmHg (lit.)
density
0.964 g/mL at 25 °C (lit.)
SMILES string
CC1(C)[C@H]2CC3OC3(C)[C@@H]1C2
InChI
1S/C10H16O/c1-9(2)6-4-7(9)10(3)8(5-6)11-10/h6-8H,4-5H2,1-3H3/t6-,7-,8?,10?/m1/s1
InChI key
NQFUSWIGRKFAHK-BGPATTHWSA-N
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Zorn et al.
Journal of biotechnology, 107(3), 255-263 (2004-01-23)
When submerged cultured Pseudomonas fluorescens NCIMB 11761 was fed-batch supplemented with alpha-pinene oxide, a rapid formation of 2,6-dimethyl-5-methylene-hept-(2Z)-enal (I) (isonovalal) was observed. Biotransformation and isomerisation of (I) to the (2E)-isomer (II) (novalal) were enhanced by Lewatit OC 1064, a macroporous
Denis Linares et al.
Bioresource technology, 99(11), 4590-4596 (2007-09-15)
The feasibility of trans-2-methyl-5-isopropylhexa-2,5-dienoic acid (novalic acid) accumulation using the alpha-pinene degradation pathway of Pseudomonas rhodesiae CIP 107491 was studied. This appeared possible by using concentrated living bacterial cells produced under oxygen limitation with alpha-pinene as sole carbon source. The
Series: 'Current issues in mutagenesis and carcinogenesis', No. 25.
P A Lefevre
Mutation research, 260(1), 5-7 (1991-05-01)
Mass spectrometric studies of DNA adducts from a reaction with terpenoids.
Wolfgang Schrader et al.
Angewandte Chemie (International ed. in English), 43(48), 6657-6660 (2004-12-14)
Hendrik Schewe et al.
Applied microbiology and biotechnology, 78(1), 55-65 (2007-12-07)
Escherichia coli BL21, expressing a quintuple mutant of P450(BM-3), oxyfunctionalizes alpha-pinene in an NADPH-dependent reaction to alpha-pinene oxide, verbenol, and myrtenol. We optimized the whole-cell biocatalyst by integrating a recombinant intracellular NADPH regeneration system through co-expression of a glucose facilitator
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service