T0581
Transglutaminase from guinea pig liver
≥1.5 units/mg protein, recombinant, expressed in E. coli
Synonym(s):
TGase
Sign Into View Organizational & Contract Pricing
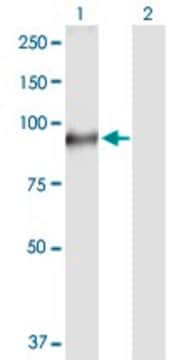
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
specific activity
≥1.5 units/mg protein
shipped in
dry ice
storage temp.
−20°C
Application
10 mM calcium chloride is used for activation of the enzyme.
Transglutaminase has been used in a study to improve quantifiable assays to fully characterize the role of transglutaminase in diseases such as Huntington′s disease and Alzheimer′s disease.Transglutaminase has also been used in a study to develop a nonradioactive dot blot assay for transglutaminase activity.
Unit Definition
One unit will catalyze the formation of 1.0 μmole of hydroxamate per minute from Nα-Z-Gln-Gly and hydroxylamine at pH 6.0 at 37 °C. (L-Glutamic acid γ-monohydroxamate is the standard.)
Physical form
Lyophilized from 10 mM NaH2PO4, 150 mM NaCl, pH 8. Contains maltodextrin.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C M Becker et al.
Archives of biochemistry and biophysics, 223(2), 381-392 (1983-06-01)
Valproic acid (dipropylacetic acid), an antiepileptic agent known to be hepatotoxic in some patients, caused inhibition of lactate gluconeogenesis, fatty acid oxidation, and fatty acid synthesis by isolated hepatocytes. The latter process was the most sensitive to valproic acid, 50%
M Kelley et al.
Biochemical pharmacology, 35(2), 289-295 (1986-01-15)
The amino acid conjugation of the phenoxyherbicides 2,4-dichlorophenoxyacetate (2,4-D) and 2,4,5-trichlorophenoxyacetate (2,4,5-T) by animals was examined at the level of the enzymes catalyzing the reactions. The phenoxyherbicides were not substrates for the bile acid conjugating system but were substrates for
Acyl-CoA esters of xenobiotic carboxylic acids as biochemically active intermediates.
H S Sherratt
Biochemical Society transactions, 13(5), 856-858 (1985-10-01)
S Kølvraa et al.
Biochemical medicine and metabolic biology, 36(1), 98-105 (1986-08-01)
Prompted by the fact that the urinary excretion of organic acids in the riboflavin-deficient rat closely mimics that found in patients with inborn errors in the acyl-CoA dehydrogenation systems, the organelle localization and the apparent kinetic constants (Km and Vmax
K D MacDermot et al.
Developmental pharmacology and therapeutics, 3(3), 150-159 (1981-01-01)
We report investigations of benzoate and glycine metabolism and glycine acyltransferase activity in rats. These studies provide insights related to the therapy and pathophysiology of human nonketotic hyperglycinemia. Liver acyltransferase activity increased sharply postnatally from low levels at birth, but
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service