All Photos(1)
About This Item
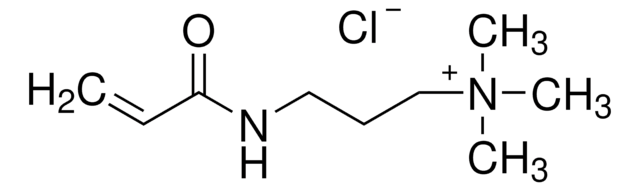
Linear Formula:
C3H6NO6SK
CAS Number:
Molecular Weight:
223.25
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
storage temp.
2-8°C
SMILES string
[K+].N[C@@H](COS([O-])(=O)=O)C(O)=O
InChI
1S/C3H7NO6S.K/c4-2(3(5)6)1-10-11(7,8)9;/h2H,1,4H2,(H,5,6)(H,7,8,9);/q;+1/p-1/t2-;/m0./s1
InChI key
LXIPCUMLLKNUSU-DKWTVANSSA-M
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Kunert
Journal of basic microbiology, 25(2), 111-118 (1985-01-01)
The dermatophyte Microsporum gypseum was cultivated on a glucose-arginine medium supplemented with five strongly acidic derivatives of cysteine (L-cysteine sulfinic acid, L-cysteic acid, L-serine-O-sulfate and taurine at a concentration of 5 mmol/l, and L-S-sulfocysteine at a concentration of 2.5 mmol/l).
K J Koller et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 5(11), 2882-2888 (1985-11-01)
[3H]-N-Acetylaspartylglutamate (NAAG) bound saturably and reversibly to crude synaptosomal rat brain membranes. Optimal binding occurred in Tris-HCl buffer, pH 7.2, at 37 degrees C using previously frozen, preincubated membranes. Saturation experiments revealed an apparent KD of 383 +/- 33 nM
H Ueno et al.
Biochemistry, 21(18), 4387-4393 (1982-08-31)
The reaction of serine O-sulfate with cytosolic aspartate aminotransferase [John, R.A., & Fasella, P. (1969) Biochemistry 8, 4477] has been reinvestigated. As in the corresponding reaction with beta-chloroalanine [Morino, Y., Osman, A.M., & Okamoto, M. (1974) J. Biol. Chem. 249
Chizuru Nagayoshi et al.
Protein and peptide letters, 16(2), 201-206 (2009-02-10)
We have successfully expressed an active human brain serine racemase (hSR) with His-tag using moderate halophile. The purified His-hSR showed high elimination and racemization activities on L-serine: the elimination activity was 2.6-fold higher than racemization activity. Both enzyme activities showed
R Contestabile et al.
European journal of biochemistry, 240(1), 150-155 (1996-08-15)
The ability of aspartate aminotransferase to catalyse beta-elimination of alpha-amino acids that have a good leaving group at C beta has been exploited in the synthesis of novel amino acids by the inclusion of appropriate nucleophiles as co-substrates. Two compounds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[2-(Acryloyloxy)ethyl]trimethylammonium chloride solution 80 wt. % in H2O, contains 600 ppm monomethyl ether hydroquinone as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/393/326/f7e19585-5431-4220-81b5-f458de6d63d0/640/f7e19585-5431-4220-81b5-f458de6d63d0.png)