MTOX1019
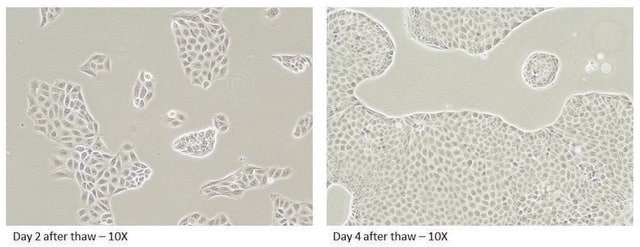
MDR1 Knockout HepaRG™ Cells
human female liver (Source Disease: Hepatocarcinoma and Hepatitis C)
About This Item
Recommended Products
product name
MDR1 Knockout HepaRG™ Cells, 1 vial
biological source
human female liver (Source Disease: Hepatocarcinoma and Hepatitis C)
form
liquid
storage temp.
−196°C
Gene Information
human ... ABCB1(5243)
General description
Application
Features and Benefits
- Sigma′s HepaRG MDR1 Knockout (KO) allows investigations of drug-transporter interactions involving MDR1 (P-glycoprotein) in the liver.
- The frame-shift mutation of MDR1 gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of transport of selective substrates in sandwich culture assay.
Quality
Legal Information
Disclaimer
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service