E4906
Enterokinase from bovine intestine
BioUltra, recombinant, expressed in E. coli, ≥20 units/mg protein, ≥95% (SDS-PAGE)
Synonym(s):
Enteropeptidase
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
eCl@ss:
32160410
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli
product line
BioUltra
Assay
≥95% (SDS-PAGE)
form
solution
specific activity
≥20 units/mg protein
mol wt
150 kDa
packaging
vial of ~0.2 unit
concentration
≥0.1 mg/mL
shipped in
wet ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Enterokinase from bovine intestine has been used in a study to assess duodenase as a potential activator of cascade digestive proteases. Enterokinase from bovine intestine has also been used in a study to investigate an inhibitor of enteropeptidases and trypsin from the bovine duodenum.
The enzyme from Sigma has been used to compare the specific activity with that of purified, recombinant bovine enterokinase (light chain) overexpressed in Escherichia coli.
Typical conditions for fusion protein cleavage:
Adjust the concentration of the fusion protein to 1.5 mg/ml and a pH between 7.0-8.0 with 500 mM Tris-HCl, pH 8.0, 2.0 mM CaCl2, and 1% Tween® 20
Add enterokinase to fusion protein solution at a ratio of ~ 0.02 units per 1 mg fusion protein and mix
Incubate reaction mixture at ~25 °C for 16 hours
Adjust the concentration of the fusion protein to 1.5 mg/ml and a pH between 7.0-8.0 with 500 mM Tris-HCl, pH 8.0, 2.0 mM CaCl2, and 1% Tween® 20
Add enterokinase to fusion protein solution at a ratio of ~ 0.02 units per 1 mg fusion protein and mix
Incubate reaction mixture at ~25 °C for 16 hours
Enterokinase is a member of the S1 peptidase family. In vivo, it is responsble for the proteolytic activation of trypsin from trypsinogen. Enterokinase is used for site specific cleavage of recombinant fusion proteins containing an accessible enterokinase recognition site for removal of affinity tags.
Biochem/physiol Actions
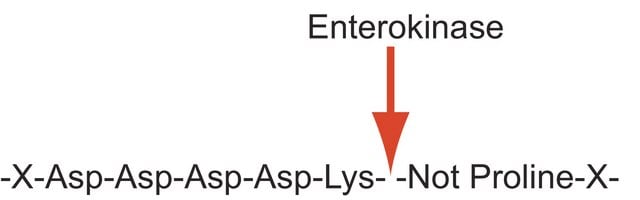
Enterokinase is a membrane bound serine protease that specifically and rapidly converts trypsinogen to trypsin, thereby, triggering the conversion of other zymogens to active enzymes. It has a molecular mass of approximately 150 kDa. The enzyme is a heterodimer, wherein, the light and the heavy chains are linked by two disulfide bridges. Native enterokinase is composed of an 800 amino acid heavy chain and a 235 amino acid light chain. It is a glycoprotein containing 35% carbohydrate. The polypeptide chain of trypsinogen is hydrolyzed only after an -(Asp)4-Lys- sequence. This cleavage site is incorporated into the FLAG tag. The FLAG® protein expression system is based on the fusion of the 8 amino acid FLAG tag to the recombinant protein of choice. Cleavage by enterokinase removes the FLAG tag from the fusion protein. The enzyme is inhibited by soybean trypsin inhibitor. Enterokinase is typically used in protein modification and amino acid sequence determination.
Physical properties
28 kDa light chain form
Unit Definition
One unit will produce 1.0 nanomole of trypsin from trypsinogen per min at pH 5.6 at 25 °C.
Physical form
supplied as a solution in 20 mM Tris-HCl, 200 mM NaCl, and 50% glycerol
Legal Information
FLAG is a registered trademark of Merck KGaA, Darmstadt, Germany
TWEEN is a registered trademark of Croda International PLC
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
X Zheng et al.
The Journal of biological chemistry, 274(3), 1596-1605 (1999-01-09)
Enteropeptidase is a heterodimeric type II membrane protein of the brush border of duodenal enterocytes. In this location, enteropeptidase cleaves and activates trypsinogen, thereby initiating the activation of other intestinal digestive enzymes. Recombinant bovine enteropeptidase was sorted directly to the
A G Mikhaĭlova et al.
Voprosy meditsinskoi khimii, 44(4), 338-346 (1998-12-10)
Enteropeptidase inhibitor (DI) was isolated from bovine duodenum during purification of this enzyme. DI was purified by affinity chromatography on immobilised trypsin. DI preparations contain two main components: DI-9 (9 kD) and DI-20 (20 kD). The N-terminal amino acid sequence
T S Zamolodchikova et al.
Bioorganicheskaia khimiia, 24(4), 300-305 (1998-06-05)
The substrate specificity of duodenase from bovine duodenum mucosa to synthetic and natural polypeptides was studied. Amino acid residues preferential for duodenase in the P1 and P2 positions of the substrate were determined. It was shown that the enzyme is
Haidong Tan et al.
Protein expression and purification, 56(1), 40-47 (2007-08-21)
The nucleotide sequence encoding bovine enterokinase light chain (EK) from Chinese northern yellow bovine was isolated. Two single-nucleotide mutations, namely, C245G and A528T were identified. The gene encoding the Pro82Arg/Glu176Asp variant of known bovine EK was fused with glutathione S-transferase
Yutetsu Kuruma et al.
Nature protocols, 10(9), 1328-1344 (2015-08-14)
Cell-free gene expression systems are biotechnological tools for the in vitro production of proteins of interest. The addition of membrane vesicles (liposomes) enables the production of membrane proteins, including those in large-molecular-weight complexes, such as the SecYEG translocon or ATP
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service