B4651
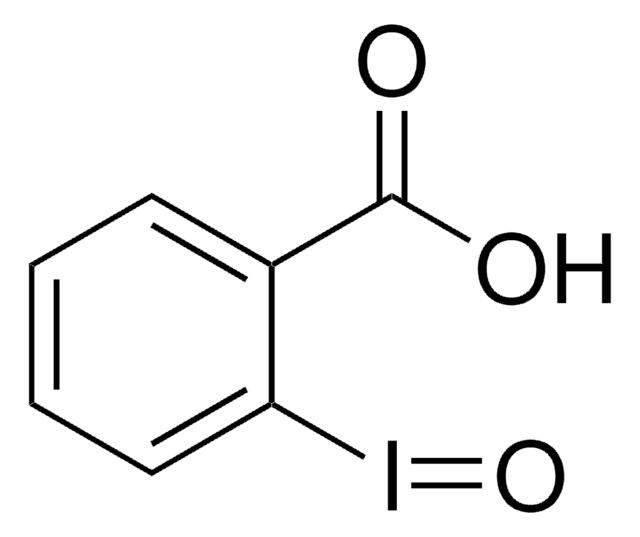
3-Bromo-3-methyl-2-(2-nitrophenylthio)-3H-indole
≥85%
Synonym(s):
2-(2-Nitrophenylsulfenyl)-3-methyl-3-bromoindolenine, 3-Bromo-3-methyl-2-(2-nitrophenylmercapto)-3H-indole, BNPS-skatol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H11BrN2O2S
CAS Number:
Molecular Weight:
363.23
Beilstein:
1491457
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
Assay
≥85%
storage temp.
−20°C
SMILES string
CC1(Br)C(Sc2ccccc2[N+]([O-])=O)=Nc3ccccc13
InChI
1S/C15H11BrN2O2S/c1-15(16)10-6-2-3-7-11(10)17-14(15)21-13-9-5-4-8-12(13)18(19)20/h2-9H,1H3
InChI key
BXTVQNYQYUTQAZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Peptide cleavage reagent specific for the carboxyl side of tryptophan residues.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V Rahali et al.
Journal of protein chemistry, 18(1), 1-12 (1999-03-11)
A comparative study of various procedures for tryptophanyl peptide bond cleavage by BNPS-skatole [2-(2-nitrophenyl)-3-methyl-3-bromoindolenine] was carried out on native and on reduced and alkylated bovine beta-lactoglobulin (BLG). The reaction yield and the composition of the derived products were studied in
Hee-Kyoung Lee et al.
Biochemical and biophysical research communications, 315(1), 1-9 (2004-03-12)
Squalene epoxidase (SE) catalyzes the conversion of squalene to (3S)-2,3-oxidosqualene. Photolabeling and site-directed mutagenesis were performed on recombinant rat SE (rrSE) in order to identify the location of the substrate-binding site and the roles of key residues in catalysis. Truncated
J E Moskaitis et al.
Neurochemical research, 11(2), 299-315 (1986-02-01)
The interactions of sodium dodecyl sulfate with a number of proteins were examined at a variety of pH values ranging from 4.8 to 11.6. The dodecyl sulfate-induced precipitation of some of these proteins was observed within a relatively limited range
The core domain of hirudin from the leech Hirudinaria manillensis. Chemical modification of a tryptophan-containing synthetic peptide analog.
F De Antoni et al.
Advances in experimental medicine and biology, 398, 627-633 (1996-01-01)
H Xue et al.
Biochemistry and cell biology = Biochimie et biologie cellulaire, 75(6), 709-715 (1997-01-01)
A concerted conformational change in Bacillus subtilis tryptophanyl-tRNA synthetase (TrpRS) was evident from previous fluorescence on the quenching of the single Trp residue Trp-92 in the 4FTrp-AMP complexed enzyme. In this study, chemical modifications of the B. subtilis TrpRS were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service