59773
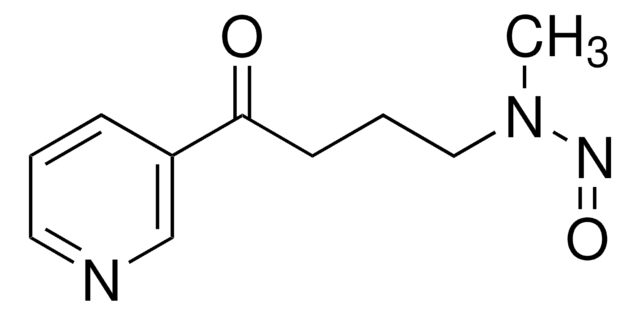
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol
analytical standard
Synonym(s):
NNAL
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H15N3O2
CAS Number:
Molecular Weight:
209.25
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥92.0% (TLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
solid phase extraction (SPE): suitable
application(s)
cleaning products
cosmetics
food and beverages
personal care
format
neat
storage temp.
2-8°C
SMILES string
CN(N=O)CCCC(O)C1=CN=CC=C1
InChI
1S/C10H15N3O2/c1-13(12-15)7-3-5-10(14)9-4-2-6-11-8-9/h2,4,6,8,10,14H,3,5,7H2,1H3
InChI key
OGRXKBUCZFFSTL-UHFFFAOYSA-N
General description
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol is one of the major metabolite of nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, which is a carcinogen responsible in inducing lung cancer in smokers.
Standard for Supelco MIP SPE cartridges. For more information request Supelco Literature T407075, T706031
Application
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S S Hecht et al.
Carcinogenesis, 18(9), 1851-1854 (1997-11-05)
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) is an important metabolite of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Using the chiral derivatizing agent, (R)-(+)-alpha-methylbenzyl isocyanate [(R)-(+)-MBIC], previous work has shown that the enantiomeric ratio of metabolically formed NNAL and its glucuronide derivative may be species dependent.
P Upadhyaya et al.
Chemical research in toxicology, 14(5), 555-561 (2001-05-23)
Nicotine and cotinine are metabolized to pyridine-N-glucuronides in humans. This suggests that the analogous metabolites of the carcinogenic nicotine-related nitrosamines N'-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) should also be formed in people exposed to these compounds via tobacco products.
Yuya Kawasaki et al.
Genes and environment : the official journal of the Japanese Environmental Mutagen Society, 42, 26-26 (2020-09-19)
Urinary nicotine and cotinine levels are often measured as biomarkers for tobacco smoke exposure. However, these biomarkers are not appropriate to evaluate the effects of quitting smoking for several days, because of their short half-lives. In this study, we focused
E M Leslie et al.
The Journal of biological chemistry, 276(30), 27846-27854 (2001-05-29)
Nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) play a crucial role in the induction of lung cancer, and NNAL-O-glucuronide formation and elimination are important steps in detoxification of these compounds. In the present study, we investigated the ATP-binding cassette
G B Smith et al.
Carcinogenesis, 20(9), 1809-1818 (1999-09-02)
Metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was characterized in human lung cells isolated from peripheral lung specimens obtained from 12 subjects during clinically indicated lobectomy. NNK biotransformation was assessed in preparations of isolated unseparated cells (cell digest), as well
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service