All Photos(1)
About This Item
Empirical Formula (Hill Notation):
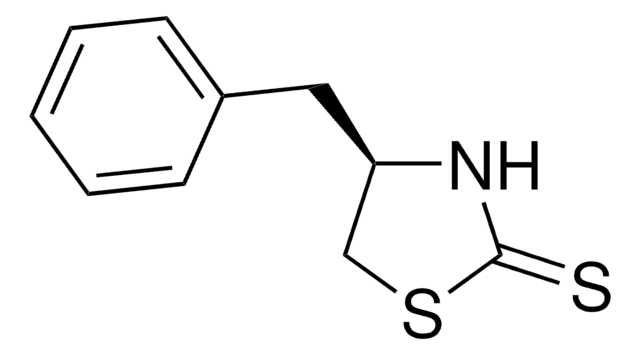
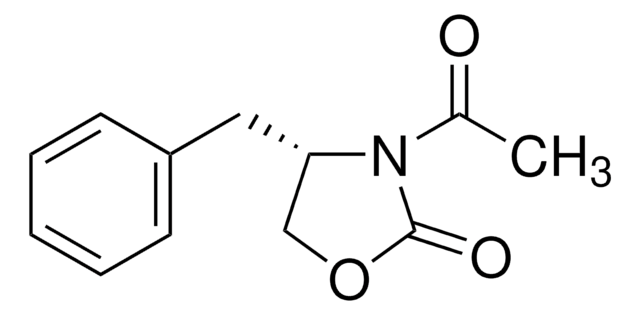
C9H9NOS
CAS Number:
Molecular Weight:
179.24
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0%
optical activity
[α]/D -75±5°, c = 0.2 in chloroform
optical purity
enantiomeric ratio: 97.0:3.0 (LC)
SMILES string
S=C1N[C@@H](CO1)c2ccccc2
InChI
1S/C9H9NOS/c12-9-10-8(6-11-9)7-4-2-1-3-5-7/h1-5,8H,6H2,(H,10,12)/t8-/m0/s1
InChI key
LVIJIGQKFDZTNC-QMMMGPOBSA-N
Application
A highly selective and efficient chiral auxiliary which can be directly reduced to its corresponding aldehyde and the chiral auxiliary by reductive cleavage with diisobutylaluminum hydride.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M T Crimmins et al.
Organic letters, 2(6), 775-777 (2001-02-07)
[formula: see text] Asymmetric aldol additions using chlorotitanium enolates of thiazolidinethione propionates proceed with high diastereoselectivity for the "Evans" or "non-Evans" syn product depending on the nature and amount of the base used. With (-)-sparteine as the base, selectivities of
Velazquez. F.; Olivo, H. F.
Current Organic Chemistry, 6, 303-303 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service