W501506
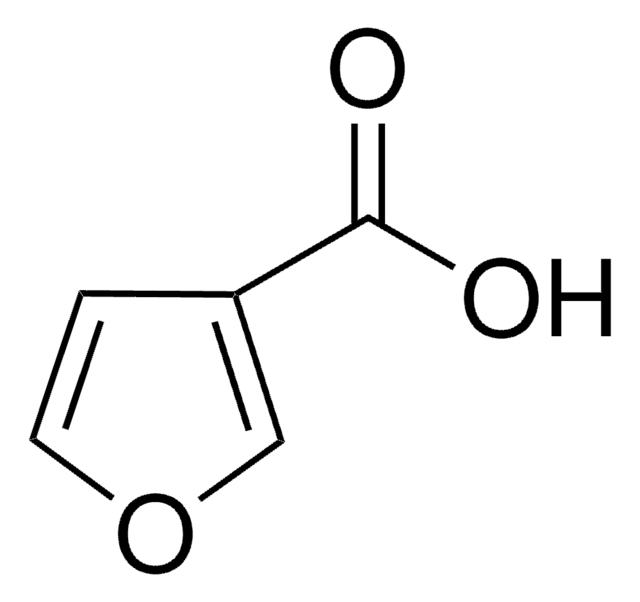
2-Furoic acid
≥97%
Synonym(s):
Furan-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H4O3
CAS Number:
Molecular Weight:
112.08
Beilstein:
110149
EC Number:
MDL number:
UNSPSC Code:
12164502
Flavis number:
13.136
Recommended Products
Assay
≥97%
bp
230-232 °C (lit.)
mp
128-132 °C (lit.)
SMILES string
OC(=O)c1ccco1
InChI
1S/C5H4O3/c6-5(7)4-2-1-3-8-4/h1-3H,(H,6,7)
InChI key
SMNDYUVBFMFKNZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Features and Benefits
Odorless
Other Notes
Download our Flavors and Fragrances Catalog to view our entire product line.
Subscribe to our Newsletter to keep up to date on our latest Flavors and Fragrances offerings.
Subscribe to our Newsletter to keep up to date on our latest Flavors and Fragrances offerings.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1C
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
282.7 °F - closed cup
Flash Point(C)
139.3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nadia Spano et al.
Talanta, 78(1), 310-314 (2009-01-29)
In this study 5-hydroxymethyl-2-furaldehyde (HMF), 2-furaldehyde, 3-furaldehyde, 2-furoic acid and 3-furoic acid are contemporarily determined in honey using a swift and direct RP-HPLC approach. The validation protocol was performed in terms of detection and quantification limits, precision (by repeatability and
K Suthindhiran et al.
Natural product research, 25(8), 834-843 (2011-04-05)
The antiviral activity of furan-2-yl acetate (C₆H₆O₃) extracted from Streptomyces VITSDK1 spp. was studied in cultured Sahul Indian Grouper Eye (SIGE) cells infected with fish nodavirus (FNV). The nodavirus infection in the SIGE cells was confirmed by reverse transcriptase-polymerase chain
H B Klinke et al.
Applied microbiology and biotechnology, 57(5-6), 631-638 (2002-01-10)
Alkaline wet oxidation (WO) (using water, 6.5 g/l sodium carbonate, and 12 bar oxygen at 195 degrees C) was used for pre-treating wheat straw (60 g/l), resulting in a hemicellulose-rich hydrolysate and a cellulose-rich solid fraction. The hydrolysate consisted of
Gema Arribas-Lorenzo et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 48(2), 644-649 (2009-12-17)
5-Hydroxymethylfurfural (HMF) is naturally formed during food processing or cooking activities, giving its ubiquity in the Western diet. HMF could be metabolised to 5-sulfooxymethylfurfural making HMF potentially harmful in an extent unknown at present. Coffee is the main exposure source.
Nancy N Nichols et al.
FEMS microbiology letters, 284(1), 52-57 (2008-05-22)
Pseudomonas putida Fu1 metabolizes furfural through a pathway involving conversion to 2-oxoglutarate, via 2-furoic acid (FA) and coenzyme A intermediates. Two P. putida transposon mutants were isolated that had impaired growth on furfural and FA, and DNA flanking the transposon
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service