D12600
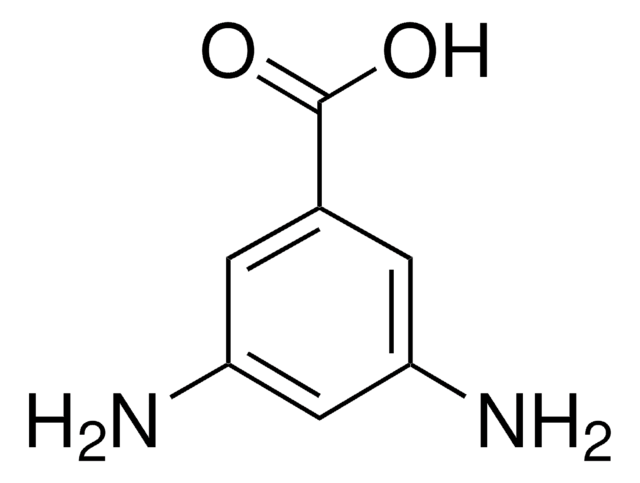
3,4-Diaminobenzoic acid
97%
Synonym(s):
4-Carboxy-o-phenylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(H2N)2C6H3CO2H
CAS Number:
Molecular Weight:
152.15
Beilstein:
775892
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
208-210 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
Nc1ccc(cc1N)C(O)=O
InChI
1S/C7H8N2O2/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3H,8-9H2,(H,10,11)
InChI key
HEMGYNNCNNODNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,4-Diaminobenzoic acid can be used to prepare:
- Schiff base derivatives by reacting with substituted aldehydes and their corresponding metal complexes.
- Poly(2,5-benzimidazole) (ABPBI) polymer by reacting with methanesulfonic acid and P2O5.
- Pt-based Schiff base complexes applicable in H2O splitting reactions.
3,4-Diaminobenzoic acid undergoes cyclocondensations to form, for example, quinoxalines and benzimidazoles.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Ram et al.
Journal of medicinal chemistry, 35(3), 539-547 (1992-02-07)
A series of methyl and ethyl 5-(alkoxycarbonyl)-1H-benzimidazole-2-carbamates (7-19) and methyl 5-carbamoyl-1H-benzimidazole-2-carbamates (24-34) have been synthesized via the reaction of an appropriate alcohol or amine with the acid chloride derivatives 6a or 6b at room temperature. Reaction of an alcohol with
L A Cooper et al.
Antonie van Leeuwenhoek, 50(1), 53-62 (1984-01-01)
The effect of various compounds on growth, melanin biosynthesis and cell differentiation was studied in a hyaline (SH25) and a pigmented (SH25B) strain of Microdochium bolleyi. Dark pigment production by the hyaline strain was induced by the presence of DOPA
Tetrahedron, 49, 9823-9823 (1993)
New 3, 4-diaminobenzoic acid Schiff base compounds and their complexes: Synthesis, characterization and thermodynamics
Mohammadi K, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 122, 179-185 (2014)
Synthesis of poly (2, 5-benzimidazole) for use as a fuel-cell membrane
Kim H, et al.
Macromolecular Rapid Communications, 25(8), 894-897 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service