All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6BrNO
CAS Number:
Molecular Weight:
188.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.555 (lit.)
bp
80 °C/12 mmHg (lit.)
density
1.453 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(Br)cn1
InChI
1S/C6H6BrNO/c1-9-6-3-2-5(7)4-8-6/h2-4H,1H3
InChI key
XADICJHFELMBGX-UHFFFAOYSA-N
Application
A building block for the β-alanine moiety of an αvβ3 antagonist and for the synthesis of a potent and selective somatostatin sst3 receptor antagonist.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nobuyoshi Yasuda et al.
The Journal of organic chemistry, 69(6), 1959-1966 (2004-04-03)
A practical preparation of an alpha(v)beta(3) antagonist is reported. The antagonist consists of three key components, a tetrahydronaphthyridine moiety, a beta-alanine moiety, and a central imidazolidone moiety. The tetrahydronaphthyridine component was prepared using two different methods, both of which relied
Negishi Cross-Coupling Reactions Catalyzed by an Aminophosphine-Based Nickel System: A Reliable and General Applicable Reaction Protocol for the High-Yielding Synthesis of Biaryls.
Gerber R and Frech CM.
Chemistry (Weinheim An Der Bergstrasse, Germany), 17(42), 11893-11904 (2011)
Tetrahedron Asymmetry, 14, 3469-3469 (2003)
A scaleable synthesis of methyl 3-amino-5-(4-fluorobenzyl)-2-pyridinecarboxylate.
Organic Process Research & Development 11.5 (2007): 899-902
Organic Process Research & Development 11.5 (2007): 899-902
Boros EE, et al.
Organic Process Research & Development, 11(5), 899-902 (2007)
Synthesis and biological evaluation of 1-(benzenesulfonamido)-2-[5-(N-hydroxypyridin-2 (1H)-one)] acetylene regioisomers: A novel class of 5-lipoxygenase inhibitors.
Chowdhury MA, et al.
Bioorganic & Medicinal Chemistry Letters, 18(14), 4195-4198 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service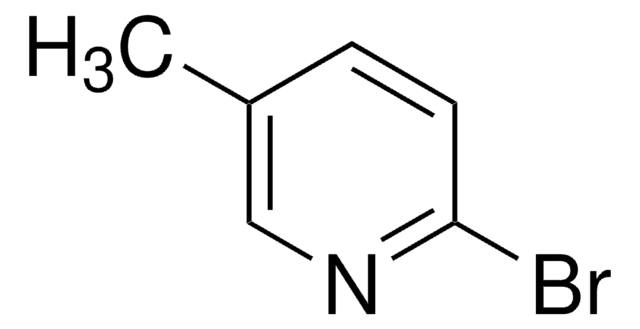







![[Pd2(dba)3] x dba Umicore](/deepweb/assets/sigmaaldrich/product/structures/150/531/11e74f1a-c256-4d30-b43d-8c299f1034b1/640/11e74f1a-c256-4d30-b43d-8c299f1034b1.png)