All Photos(1)
About This Item
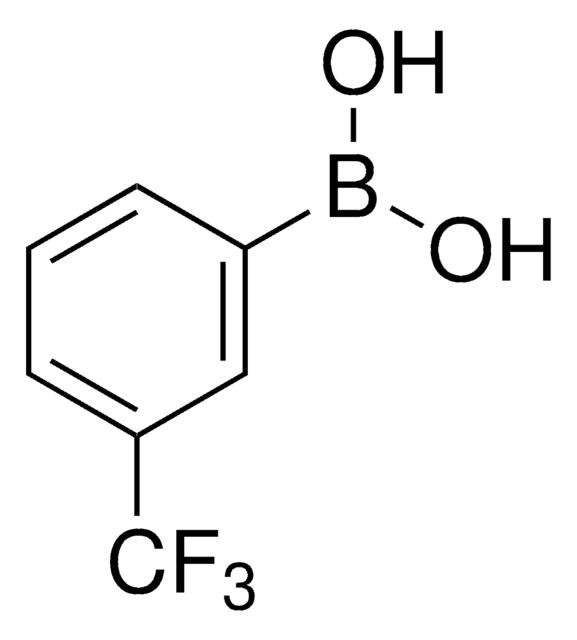
Linear Formula:
CF3C6H4B(OH)2
CAS Number:
Molecular Weight:
189.93
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
form
solid
mp
111-114 °C (lit.)
SMILES string
OB(O)c1ccccc1C(F)(F)F
InChI
1S/C7H6BF3O2/c9-7(10,11)5-3-1-2-4-6(5)8(12)13/h1-4,12-13H
InChI key
JNSBEPKGFVENFS-UHFFFAOYSA-N
Application
2-(Trifluoromethyl)phenylboronic acid can be used as a reactant:
- In Suzuki-coupling reactions to prepare 2-trifluoromethyl aryl or heteroaryl derivatives.
- To synthesize 4-(2-trifluoromethyl)phenylpyrrolo[2,3-d]pyrimidine as a potential antagonist of corticotropin-releasing hormone.
- To prepare 2-nitro-6-(trifluoromethyl)phenylboronic acid by nitration reaction.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Studies of palladium-catalyzed coupling reactions for preparation of hindered 3-arylpyrroles relevant to (-)-rhazinilam and its analogues
Ghosez L, et al.
Canadian Journal of Chemistry, 79(11), 1827-1839 (2001)
Efficient synthetic approach to heterocycles possessing the 3, 3-disubstituted-2, 3-dihydrobenzofuran skeleton via diverse palladium-catalyzed tandem reactions
Szlosek-Pinaud M, et al.
Tetrahedron, 63(16), 3340-3349 (2007)
Functionalization of pyrrolo [2, 3-d] pyrimidine by palladium-catalyzed cross-coupling reactions
Tumkevicius, S and Dodonova, J
Chemistry of Heterocyclic Compounds, 48(2), 258-279 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service