All Photos(1)
About This Item
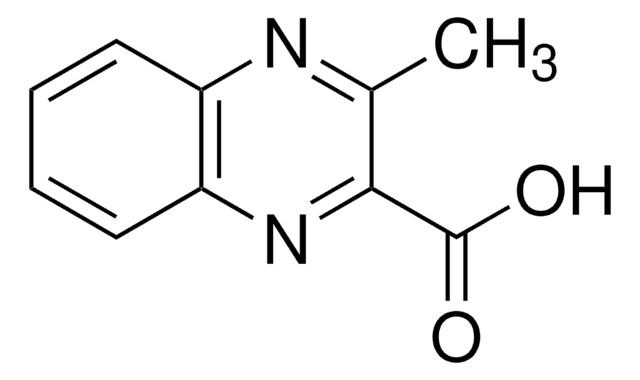
Empirical Formula (Hill Notation):
C9H6N2O2
CAS Number:
Molecular Weight:
174.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
208 °C (dec.) (lit.)
SMILES string
OC(=O)c1cnc2ccccc2n1
InChI
1S/C9H6N2O2/c12-9(13)8-5-10-6-3-1-2-4-7(6)11-8/h1-5H,(H,12,13)
InChI key
UPUZGXILYFKSGE-UHFFFAOYSA-N
General description
Linear and Freundlich adsorption isotherm coefficient of 2-quinoxalinecarboxylic acid has been evaluated.
Application
2-Quinoxalinecarboxylic acid has been used in the preparation of:
- N-(2-quinoxaloyl)-α-amino acids
- bisquinoxaloyl (bisquinoxalinecarbonyl) derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Joshua A Hagen et al.
Sensors (Basel, Switzerland), 11(7), 6645-6655 (2011-12-14)
Zinc oxide field effect transistors (ZnO-FET), covalently functionalized with single stranded DNA aptamers, provide a highly selective platform for label-free small molecule sensing. The nanostructured surface morphology of ZnO provides high sensitivity and room temperature deposition allows for a wide
Alex P Praseuth et al.
Biotechnology progress, 24(1), 134-139 (2008-01-05)
Proficient production of the antitumor agent triostin A was developed using engineered Escherichia coli (E. coli). The bacterium played host to 15 genes that encode integral biosynthetic proteins which were identified and cloned from Streptomyces lasaliensis. In this study, triostin
Kento Koketsu et al.
Organic letters, 8(21), 4719-4722 (2006-10-06)
[reaction: see text] Little is known about how quinoxaline-2-carboxylic acid (QC) is synthesized in nature. On the basis of analysis of echinomycin biosynthetic gene clusters as well as feeding experiments with labeled precursors, we have proposed a biosynthetic pathway to
M Rutalj et al.
Food additives and contaminants, 13(8), 879-882 (1996-11-01)
The concentration of quinoxaline-2-carboxylic acid (QCA) determined by HPLC after alkaline hydrolysis of liver and muscle of swine, ranged from < 3 ng/g to 45.3 ng/g in liver, and from < 3 ng/g to 10.8 ng/g in muscle samples. After
Synthetic Approaches to Quinoxaline Antibiotics. Synthesis of Bisquinoxaloyl Derivatives1a.
Koppel HC, et al.
The Journal of Organic Chemistry, 28(4), 1119-1122 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service