All Photos(1)
About This Item
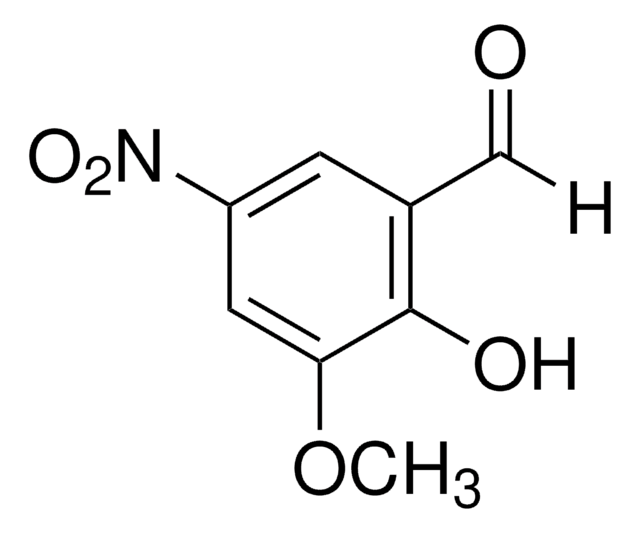
Linear Formula:
(O2N)2C6H2-2-(OH)CHO
CAS Number:
Molecular Weight:
212.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
68-70 °C (lit.)
SMILES string
Oc1c(C=O)cc(cc1[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C7H4N2O6/c10-3-4-1-5(8(12)13)2-6(7(4)11)9(14)15/h1-3,11H
InChI key
FLJXIBHYDIMYRS-UHFFFAOYSA-N
Application
3,5-Dinitrosalicylaldehyde has been used in the preparation of:
- salicyldimine ligand via Schiff base condensation with allyl-substituted aniline
- 3-hydroxycoumarins
- chromogenic proteinase substrates
- Ruthenium(II) chiral Schiff base complexes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dao Zhang et al.
Chemical communications (Cambridge, England), (6)(6), 574-575 (2002-07-18)
A new family of self-immobilized ethylene polymerization catalysts, derived from neutral, single-component salicylaldiminato phenyl nickel complexes, is described.
Synthesis, physico-chemical studies and solvent-dependent enantioselective epoxidation of 1, 2-dihydronaphthalene catalysed by chiral Ruthenium (II) Schiff base complexes
Kureshy RI, et al.
J. Mol. Catal. A: Chem., 150(1), 163-173 (1999)
Notes-3-Hydroxycoumarins.
Trivedi K and Sethan S.
The Journal of Organic Chemistry, 25(10), 1817-1819 (1960)
N G Gallegos et al.
Journal of biochemical and biophysical methods, 33(1), 31-41 (1996-10-15)
To search for new proteinases in Bacillus subtilis we have developed a general method for synthesizing chromogenic proteinase substrates using 3,5-dinitrosalicylaldehyde (DNSA). Hammersten casein and soluble protein from extracts from B. subtilis cells were labeled with DNSA in the presence
Ryan J DiRisio et al.
Dalton transactions (Cambridge, England : 2003), 46(31), 10418-10425 (2017-07-27)
Two cobalt(iii) complexes containing inexpensive Schiff-base ligands have been found to be active for proton reduction at low overpotentials. The dinitro and tetranitro derivatized Schiff-base complexes show catalytic activity at -0.96 V and -1.1 V vs. Fc
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service