273112
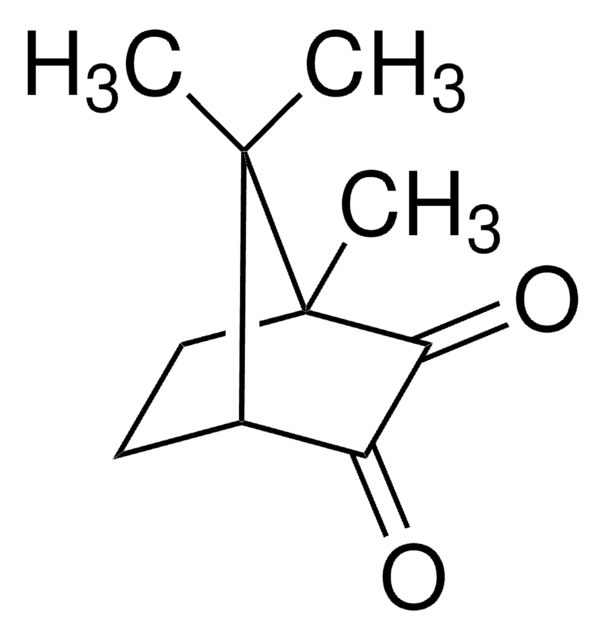
(1R,E)-(+)-Camphorquinone 3-oxime
99%
Synonym(s):
(1R,E)-(+)-2,3-Bornanedione 3-oxime, anti-(1R)-(+)-Camphorquinone 3-oxime
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H15NO2
CAS Number:
Molecular Weight:
181.23
Beilstein:
3200377
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
optical activity
[α]25/D +200°, c = 1 in ethanol
mp
153-156 °C (lit.)
functional group
ketone
oxime
SMILES string
[H][C@@]12CC[C@@](C)(C(=O)\C1=N/O)C2(C)C
InChI
1S/C10H15NO2/c1-9(2)6-4-5-10(9,3)8(12)7(6)11-13/h6,13H,4-5H2,1-3H3/b11-7-/t6-,10+/m1/s1
InChI key
YRNPDSREMSMKIY-HAKKTOSXSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(1R,E)-(+)-Camphorquinone 3-oxime can be used to prepare chiral camphor-oxazoline auxiliary, which is used to blend diastereoselectivity and isomer separability for the efficient synthesis of bicuculline, egenine, corlumine, and corytensine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
1-Magnesiotetrahydroisoquinolyloxazolines as Chiral Nucleophiles in Stereoselective Additions to Aldehydes: Auxiliary Optimization, Asymmetric Synthesis of (+)-Corlumine,(+)-Bicuculline,(+)-Egenine, and (+)-Corytensine, and Preliminary 13C NMR Studies of 1-Lithio-and 1-Magnesiotetrahydroisoquinolyloxazolines
Gawley RE and Z Pingsheng
The Journal of Organic Chemistry, 61(23), 8103- 8112 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service