All Photos(1)
About This Item
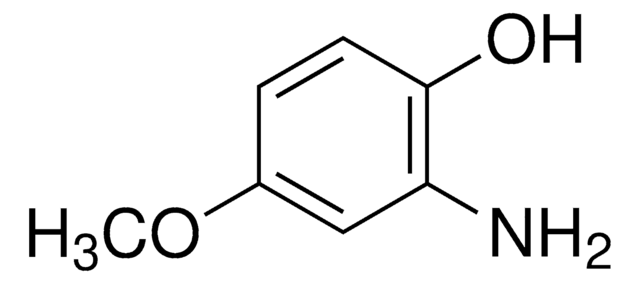
Linear Formula:
(CH3)3CC6H3(NH2)OH
CAS Number:
Molecular Weight:
165.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
160-163 °C (lit.)
SMILES string
CC(C)(C)c1ccc(O)c(N)c1
InChI
1S/C10H15NO/c1-10(2,3)7-4-5-9(12)8(11)6-7/h4-6,12H,11H2,1-3H3
InChI key
RPJUVNYXHUCRMG-UHFFFAOYSA-N
Related Categories
Application
2-Amino-4-tert-butylphenol can be used as a reactant to prepare:
- 4-tert-butyl-2-[(pyridylmethylene)amino]phenol intermediates, which are used to synthesize biologically important 2-(pyridyl)benzoxazole derivatives.
- Prolinamide phenols, as efficient hydrophobic organocatalysts for direct asymmetric aldol reaction aldehydes and ketones in water.
- N-(2-hydroxy-4-tert-butylphenyl)-acetamide, a key intermediate to prepare uranylsalophene derivatives which can be used as selective receptors in anion sensitive membrane sensors.
- Poly(2-amino-4-tert-butylphenol) [poly(2A-4TBP)] by electrochemical or chemical oxidative polymerization reaction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The chemical and electrochemical oxidative polymerization of 2-amino-4-tert-butylphenol
Abidi M, et al.
Electrochimica Acta, 212, 958-965 (2016)
Synthesis of 2-(2-, 3-, and 4-pyridyl) benzoxazoles by the reaction of phenolic Schiff bases with thianthrene cation radical.
Park MS, et al.
Journal of Heterocyclic Chemistry, 39(6), 1279-1282 (2002)
Neutral anion receptors: synthesis and evaluation as sensing molecules in chemically modified field effect transistors.
Antonisse MMG, et al.
The Journal of Organic Chemistry, 62(26), 9034-9038 (1997)
Rationally designed 4-phenoxy substituted prolinamide phenols organocatalyst for the direct aldol reaction in water
Z Shu-peng, et al.
Tetrahedron Letters, 50(11), 1173-1176 (2009)
Rationally designed 4-phenoxy substituted prolinamide phenols organocatalyst for the direct aldol reaction in water.
Zhang S-P, et al.
Tetrahedron Letters, 50(11), 1173-1176 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![4-(DIMETHYLAMINO)BENZALDEHYDE [4-(DIMETHYLAMINO)BENZYLIDENE]HYDRAZONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/268/291/5232c253-7dd7-435c-b094-6d334239d9fb/640/5232c253-7dd7-435c-b094-6d334239d9fb.png)