The product impurities are not identified or quantified. Please see the Specification Sheet: https://www.sigmaaldrich.com/specification-sheets/360/076/159034-BULK_______ALDRICH__.pdf
Select a Size
Select a Size
About This Item
Recommended Products
Quality Level
Assay
98%
mp
88-90 °C (lit.)
functional group
amine
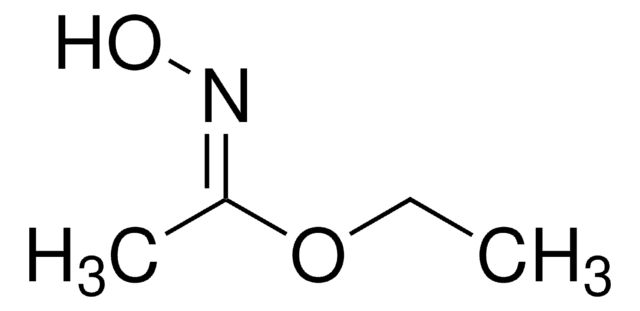
SMILES string
CC(NO)=O
InChI
1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4)
InChI key
RRUDCFGSUDOHDG-UHFFFAOYSA-N
Gene Information
human ... CA2(760) , MMP3(4314)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
Hello, Could you tell me the percentage of impurities and define specifically which impurities we can find in this reagent ? Thank you,
1 answer-
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service