141569
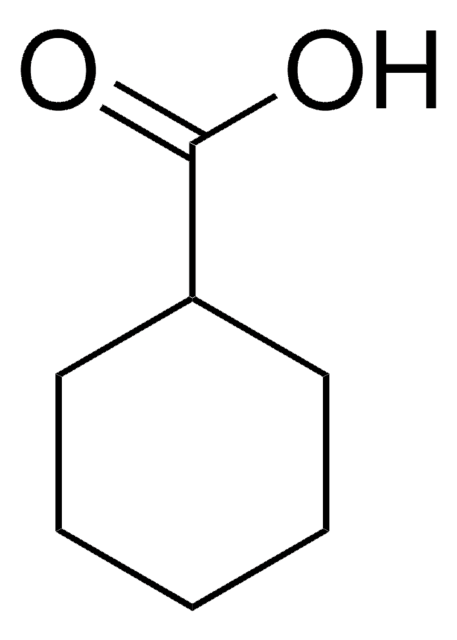
1-(4-Chlorophenyl)-1-cyclopentanecarboxylic acid
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4C5H8CO2H
CAS Number:
Molecular Weight:
224.68
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
form
solid
mp
162-164 °C (lit.)
functional group
carboxylic acid
chloro
SMILES string
OC(=O)C1(CCCC1)c2ccc(Cl)cc2
InChI
1S/C12H13ClO2/c13-10-5-3-9(4-6-10)12(11(14)15)7-1-2-8-12/h3-6H,1-2,7-8H2,(H,14,15)
InChI key
QJNFJEMGWIQMJT-UHFFFAOYSA-N
Biochem/physiol Actions
1-(4-Chlorophenyl)-1-cyclopentanecarboxylic acid reacts with diorganotin(IV) oxide or dichloride to yield organotin(IV) complexes having antitumor activities against various human cancer cell lines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xianmei Shang et al.
Inorganic chemistry, 50(17), 8158-8167 (2011-07-29)
The organotin(IV) compounds [Me(2)Sn(L)(2)] (1), [Et(2)Sn(L)(2)] (2), [(n)Bu(2)Sn(L)(2)] (3), [(n)Oct(2)Sn(L)(2)] (4), [Ph(2)Sn(L)(2)] (5), and [PhOSnL](6) (6) have been synthesized from the reactions of 1-(4-chlorophenyl)-1-cyclopentanecarboxylic acid (HL) with the corresponding diorganotin(IV) oxide or dichloride. They were characterized by IR and multinuclear
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service