119474
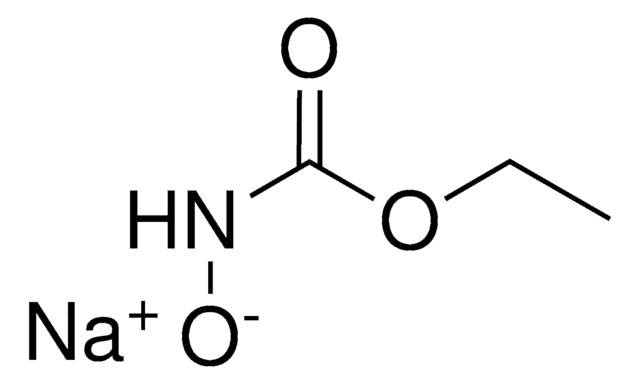
N-Hydroxyurethane
Synonym(s):
N-Carbethoxyhydroxylamine, Ethyl N-hydroxycarbamate, Hydroxycarbamic acid ethyl ester, NSC 71045, NSC 83629
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HONHCOOCH2CH3
CAS Number:
Molecular Weight:
105.09
Beilstein:
1747529
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.445 (lit.)
bp
113-116 °C/3 mmHg (lit.)
storage temp.
−20°C
SMILES string
CCOC(=O)NO
InChI
1S/C3H7NO3/c1-2-7-3(5)4-6/h6H,2H2,1H3,(H,4,5)
InChI key
VGEWEGHHYWGXGG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Hydroxyurethane was used to synthesize N-methyl-O-benzylhydroxylamine and N-isopropyl-O-methylhydroxylamine.
Reactant involved in:
- Synthesis of molecules used for intermolecular Sharpless aminohydroxylation reactions
- Intermolecular ortho-C-H amidation of anilides
- Cinchona alkaloid-catalyzed asymmetric cycloaddition
- Allylic arylation
Biochem/physiol Actions
N-Hydroxyurethane causes the chromosomal fragmentation at millimolar concentrations and cell toxicity in cultured normal human leukocytes.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kinetic applications of electron paramagnetic resonance spectroscopy. 28. N-Alkoxy-N-alkylamino, N-alkoxyamino, and N-alkoxyanilino radicals.
Kaba RA and Ingold KU
Journal of the American Chemical Society, 98(23), 7375-7380 (1976)
Induction of chromosome breaks in cultured normal human leukocytes by potassium arsenite, hydroxyurea and related compounds.
J J Oppenheim et al.
Cancer research, 25(7), 980-985 (1965-08-01)
P M Weiss et al.
Biochemistry, 23(19), 4346-4350 (1984-09-11)
The true substrate for the pyruvate kinase catalyzed phosphorylation of hydroxylamine at high pH which is activated by bicarbonate is shown to be N-hydroxycarbamate, since a lag is seen when the reaction is started by the addition of bicarbonate or
Katsuhisa Sakano et al.
Free radical biology & medicine, 33(5), 703-714 (2002-09-05)
Carcinogenic urethane (ethyl carbamate) forms DNA adduct via epoxide, whereas carcinogenic methyl carbamate can not. To clarify a mechanism independent of DNA adduct formation, we examined DNA damage induced by N-hydroxyurethane, a urethane metabolite, using 32P-5'-end-labeled DNA fragments. N-hydroxyurethane induced
S Rossberger et al.
Mutation research, 145(3), 201-207 (1985-05-01)
N-Hydroxyurea and two structurally related compounds, acetohydroxamic acid and N-hydroxyurethane, were investigated for their potential to induce DNA repair synthesis in primary rat hepatocyte cultures. Repair was determined as repair replication by means of the bromodeoxyuridine density-shift method and, in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service