91255
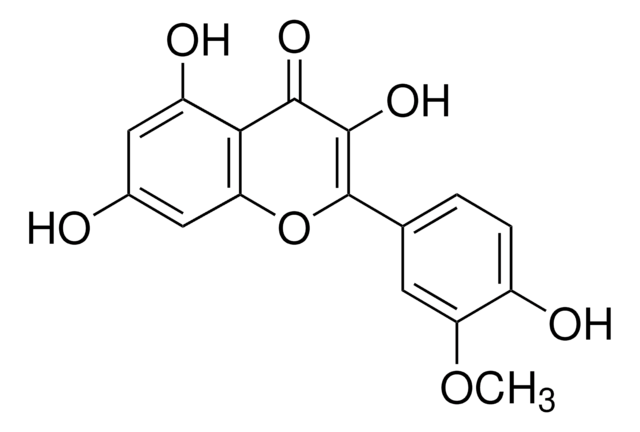
Myricitrin
≥99.0% (HPLC)
Sinónimos:
3,3′,4′,5,5′,7-Hexahydroxyflavone 3-O-rhamnoside, Myricetin 3-O-α-L-rhamnopyranoside, Myricetin 3-O-rhamnoside, Myricitroside
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥99.0% (HPLC)
Formulario
powder
técnicas
HPLC: suitable
color
light yellow
temp. de almacenamiento
2-8°C
cadena SMILES
C[C@@H]1O[C@@H](OC2=C(Oc3cc(O)cc(O)c3C2=O)c4cc(O)c(O)c(O)c4)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C21H20O12/c1-6-14(26)17(29)18(30)21(31-6)33-20-16(28)13-9(23)4-8(22)5-12(13)32-19(20)7-2-10(24)15(27)11(25)3-7/h2-6,14,17-18,21-27,29-30H,1H3/t6-,14-,17+,18+,21-/m0/s1
Clave InChI
DCYOADKBABEMIQ-OWMUPTOHSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Acciones bioquímicas o fisiológicas
Envase
Otras notas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico