21872
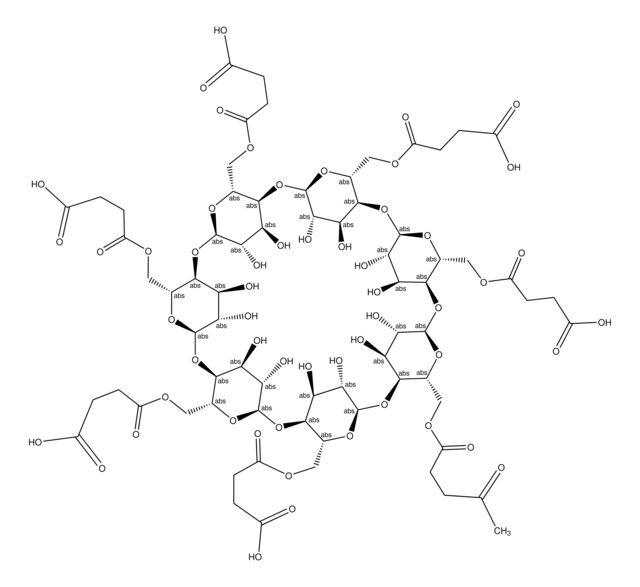
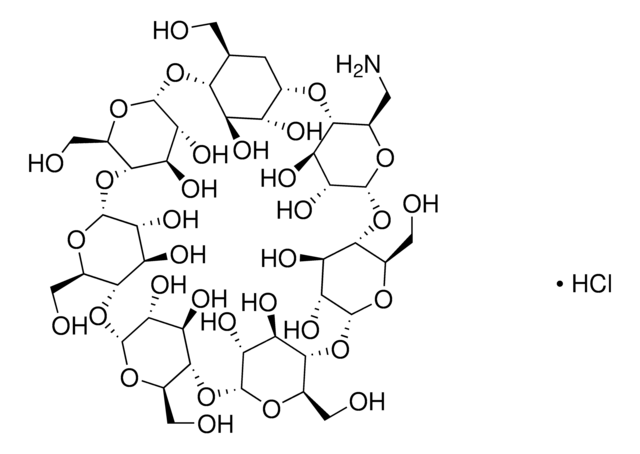
(2-Carboxyethyl)-β-cyclodextrin sodium salt
About This Item
Productos recomendados
Formulario
solid
actividad óptica
[α]20/D +129±6°, c = 1% in H2O
impurezas
≤10% acidic form
~5% water
solubilidad
H2O: 50 mg/mL, clear, colorless
temp. de almacenamiento
room temp
Descripción general
Aplicación
- As a chiral selector for the enantioseparation of cinacalcet impurities[2] and fenamiphos pesticide[3] by capillary zone electrophoresis and 31P nuclear magnetic resonance spectroscopy (31P NMR), respectively.
- To form an inclusion complex with carvacrol for subsequent use in the antibacterial multilayer construction with chitosan, loaded onto poly(L-lactic acid) (PLLA).[1]
Nota de análisis
Otras notas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico