07551
Vanillylidenacetone
≥98.5%
Sinónimos:
4-(4-Hydroxy-3-methoxyphenyl)-3-buten-2-one
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C11H12O3
Número de CAS:
Peso molecular:
192.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥98.5%
color
yellow
mp
125-130 °C
SMILES string
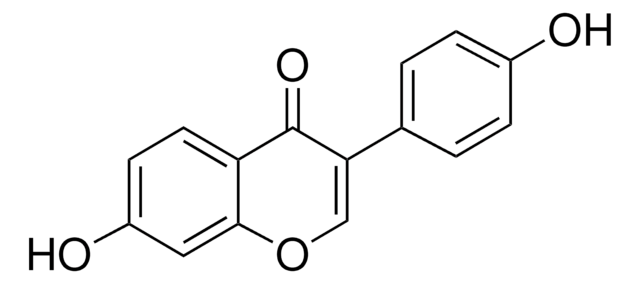
COc1cc(\C=C\C(C)=O)ccc1O
InChI
1S/C11H12O3/c1-8(12)3-4-9-5-6-10(13)11(7-9)14-2/h3-7,13H,1-2H3/b4-3+
InChI key
AFWKBSMFXWNGRE-ONEGZZNKSA-N
Gene Information
human ... APP(351)
¿Está buscando productos similares? Visita Guía de comparación de productos
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Pharmacological actions and acute toxicity of methyl- and phenyl-3-methoxy-4-hydroxy styryl ketones.
G B Singh et al.
Arzneimittel-Forschung, 37(6), 708-712 (1987-06-01)
Some pharmacological actions and acute toxicity effects of methyl- and phenyl-3-methoxy-4-hydroxy styryl ketones have been described in experimental animals. The compounds antagonised the contractions evoked by a variety of agonists on several smooth muscle preparations in vitro. They produced inhibitory
I Rahath Kubra et al.
Journal of food science, 78(1), M64-M69 (2013-01-03)
The efficacy of Dehydrozingerone (DZ; dehydroderivative of zingerone) as an antifungal agent and its mode of action against food spoilage fungal pathogens was studied and presented. DZ is a constituent of ginger (Zingiber officinale rhizomes) and structural half analogue of
D V Rajakumar et al.
Die Pharmazie, 49(7), 516-519 (1994-07-01)
The antioxidant property of dehydrozingerone and its analogs were investigated in the ferric-ascorbate induced lipid peroxidation model in rat brain homogenate. All the non phenolic compounds were either inactive or less active, while phenolic compounds with substitution at both meta
D V Rajakumar et al.
Biochemical pharmacology, 46(11), 2067-2072 (1993-12-03)
The antioxidant properties of three related compounds, dehydrozingerone, isoeugenol and eugenol, were investigated using various models. Isoeugenol was found to be the most active in inhibiting ferrous-ion-, ferric-ion- and cumene-hydroperoxide-induced lipid peroxidation in rat brain homogenates. These compounds also showed
Yizhen Liu et al.
Journal of cardiovascular pharmacology, 52(5), 422-429 (2008-11-27)
Growth factor and oxidative stress-mediated migration and proliferation of vascular smooth muscle cells (VSMCs) play a key role in the pathogenesis of atherosclerosis. The objective of this study was to assess the ability of dehydrozingerone, a structural analog of curcumin
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico