17-677
ChIPAb+ Dimethyl-Histone H3 (Lys4) - ChIP Validated Antibody and Primer Set
clone CMA303, from mouse
Sinónimos:
H3K4me2, Histone H3 (di methyl K4)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.52
Productos recomendados
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
clone
CMA303, monoclonal
species reactivity
human, vertebrates
manufacturer/tradename
ChIPAb+
Upstate®
technique(s)
ChIP: suitable (ChIP-seq)
dot blot: suitable
multiplexing: suitable
western blot: suitable
isotype
IgG2bκ
NCBI accession no.
UniProt accession no.
shipped in
dry ice
General description
All ChIPAb+ antibodies are individually validated for chromatin precipitation, every lot, every time. Each ChIPAb+ antibody set includes control primers (tested every lot by qPCR) to biologically validate your IP results in a locus-specific context. The qPCR protocol and primer sequences are provided, allowing researchers to validate ChIP protocols when using our antibody in their chromatin context. Each set also includes a negative control antibody to ensure specificity of the ChIP reaction.
The ChIPAb+ Dimethyl-Histone H3 (Lys4) set includes the Anti-dimethyl-Histone H3 (Lys4) antibody, a negative control antibody (purified Mouse IgG), and qPCR primers which amplify a 213 bp region within the coding region of the human GAPDH gene. The dimethyl-histone H3 (Lys4) and negative control antibodies are supplied in a scalable "per ChIP" reaction size and can be used to functionally validate the precipitation of dimethyl-histone H3 (Lys4)-associated chromatin.
The ChIPAb+ Dimethyl-Histone H3 (Lys4) set includes the Anti-dimethyl-Histone H3 (Lys4) antibody, a negative control antibody (purified Mouse IgG), and qPCR primers which amplify a 213 bp region within the coding region of the human GAPDH gene. The dimethyl-histone H3 (Lys4) and negative control antibodies are supplied in a scalable "per ChIP" reaction size and can be used to functionally validate the precipitation of dimethyl-histone H3 (Lys4)-associated chromatin.
Specificity
Recognizes histone H3, Mr 17 kDa, dimethylated at lysine 4.
The peptide sequence is identical in a wide range of animal and plant species, so broad cross-reactivity is expected.
Immunogen
Epitope: a.a. 1-12
The dimethyl-histone H3 (Lys4) purified antibody is made against a synthetic peptide (dimethylated at Lys4) corresponding to amino acids 1-12 of histone H3.
Application
Chromatin Immunoprecipitation:
Sonicated chromatin prepared from HeLa cells (2 X 106 cell equivalents per IP) was subjected to chromatin immunoprecipitation using 2 µg of either normal mouse IgG, or Anti-dimethyl-Histone H3 (Lys4) antibody and the Magna ChIP G Kit (Cat. #17-611). Successful immunoprecipitation of dimethyl-histone H3 (Lys4)-associated DNA fragments was verified by qPCR using β-globin ChIP Primers versus GAPDH Coding primers (Please see figures). Data is presented as percent input of each IP sample relative to input chromatin for each antibody with the indicated primers.
Please refer to the EZ-Magna G ChIP (Cat. #17-408) or EZ-ChIP (Cat. #17-371) protocol for experimental details.
ChIP-seq Analysis:
Chromatin immunoprecipitation was performed using the Magna ChIP HiSens kit (cat# 17-10460), 2 µg Anti-dimethyl-Histone H3 (Lys4) antibody (cat# 17-677), 20 µL Protein A/G beads, and 1e6 crosslinked HeLa cell chromatin followed by DNA purification using magnetic beads. Libraries were prepared from Input and ChIP DNA samples using standard protocols with Illumina barcoded adapters, and analyzed on Illumina HiSeq instrument. An excess of sixteen million reads from FastQ files were mapped using Bowtie (http://bowtie-bio.sourceforge.net/manual.shtml) following TagDust (http://genome.gsc.riken.jp/osc/english/dataresource/) tag removal. Peaks were identified using MACS (http://luelab.dfci.harvard.edu/MACS/), with peaks and reads visualized as a custom track in UCSC Genome Browser (http://genome.ucsc.edu) from BigWig and BED files. The highest 25% of peaks identified in the 04-790 and 17-677 datasets showed 92 and 90% overlap with peaks identified in the ENCODE H3K4me2 BROAD Histone track for HeLa S3.
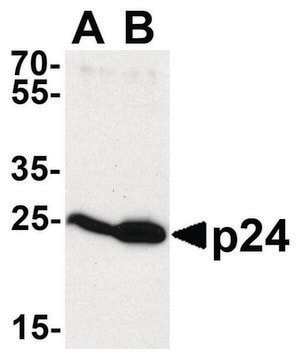
Western Blot Analysis:
Recombinant Histone H3 (Lane 1) and HeLa acid extract (Lane 2) were resolved by electrophoresis, transferred to PVDF membrane and probed with Anti-dimethyl Histone H3 (Lys4) (2 μg/mL).
Proteins were visualized using a goat anti-mouse secondary antibody conjugated to HRP (Cat. #AP124P) and a chemiluminescence detection system.
Dot Blot Analysis:
Absurance Histone H3 Antibody Specificity Array (Cat. No. 16-667) and Absurance Histone H2A, H2B, H4 Antibody Specificity Array (Cat. No. 16-665), which contain histone peptides with various modifications were probed with Anti-dimethyl H3 (Lys4) at 2.0ug/mL (1:500) dilution. Proteins were visualized using a Donkey anti-mouse IgG conjugated to HRP and a chemiluminescence detection system.
Beadlyte Histone Peptide Specificity Assay:
0.15 μg/ml of purified anti-dimethyl-Histone H3 (Lys4),
clone CMA303 was incubated with a cocktail of microspheres
conjugated to histone H3 peptides with the following modifications:
1. unmodified H3 (K4)
2. monomethyl H3 (K4)
3. dimethyl H3 (K4)
4. trimethyl H3 (K4)
5. trimethy H3 (K27)
Unbound antibody was then removed by filtration.
Peptide antibody complexes were incubated with a
PE-conjugated anti-mouse secondary antibody.
Fluorescence was read on a Luminex 100
instrument. Median Fluorescence intensity (MFI) is plotted.
Sonicated chromatin prepared from HeLa cells (2 X 106 cell equivalents per IP) was subjected to chromatin immunoprecipitation using 2 µg of either normal mouse IgG, or Anti-dimethyl-Histone H3 (Lys4) antibody and the Magna ChIP G Kit (Cat. #17-611). Successful immunoprecipitation of dimethyl-histone H3 (Lys4)-associated DNA fragments was verified by qPCR using β-globin ChIP Primers versus GAPDH Coding primers (Please see figures). Data is presented as percent input of each IP sample relative to input chromatin for each antibody with the indicated primers.
Please refer to the EZ-Magna G ChIP (Cat. #17-408) or EZ-ChIP (Cat. #17-371) protocol for experimental details.
ChIP-seq Analysis:
Chromatin immunoprecipitation was performed using the Magna ChIP HiSens kit (cat# 17-10460), 2 µg Anti-dimethyl-Histone H3 (Lys4) antibody (cat# 17-677), 20 µL Protein A/G beads, and 1e6 crosslinked HeLa cell chromatin followed by DNA purification using magnetic beads. Libraries were prepared from Input and ChIP DNA samples using standard protocols with Illumina barcoded adapters, and analyzed on Illumina HiSeq instrument. An excess of sixteen million reads from FastQ files were mapped using Bowtie (http://bowtie-bio.sourceforge.net/manual.shtml) following TagDust (http://genome.gsc.riken.jp/osc/english/dataresource/) tag removal. Peaks were identified using MACS (http://luelab.dfci.harvard.edu/MACS/), with peaks and reads visualized as a custom track in UCSC Genome Browser (http://genome.ucsc.edu) from BigWig and BED files. The highest 25% of peaks identified in the 04-790 and 17-677 datasets showed 92 and 90% overlap with peaks identified in the ENCODE H3K4me2 BROAD Histone track for HeLa S3.
Western Blot Analysis:
Recombinant Histone H3 (Lane 1) and HeLa acid extract (Lane 2) were resolved by electrophoresis, transferred to PVDF membrane and probed with Anti-dimethyl Histone H3 (Lys4) (2 μg/mL).
Proteins were visualized using a goat anti-mouse secondary antibody conjugated to HRP (Cat. #AP124P) and a chemiluminescence detection system.
Dot Blot Analysis:
Absurance Histone H3 Antibody Specificity Array (Cat. No. 16-667) and Absurance Histone H2A, H2B, H4 Antibody Specificity Array (Cat. No. 16-665), which contain histone peptides with various modifications were probed with Anti-dimethyl H3 (Lys4) at 2.0ug/mL (1:500) dilution. Proteins were visualized using a Donkey anti-mouse IgG conjugated to HRP and a chemiluminescence detection system.
Beadlyte Histone Peptide Specificity Assay:
0.15 μg/ml of purified anti-dimethyl-Histone H3 (Lys4),
clone CMA303 was incubated with a cocktail of microspheres
conjugated to histone H3 peptides with the following modifications:
1. unmodified H3 (K4)
2. monomethyl H3 (K4)
3. dimethyl H3 (K4)
4. trimethyl H3 (K4)
5. trimethy H3 (K27)
Unbound antibody was then removed by filtration.
Peptide antibody complexes were incubated with a
PE-conjugated anti-mouse secondary antibody.
Fluorescence was read on a Luminex 100
instrument. Median Fluorescence intensity (MFI) is plotted.
Research Category
Epigenetics & Nuclear Function
Epigenetics & Nuclear Function
Research Sub Category
Chromatin Biology
Chromatin Biology
This ChIPAb+ Dimethyl-Histone H3 (Lys4) -ChIP Validated Antibody & Primer Set conveniently includes the antibody & the specific control PCR primers.
Packaging
25 assays per kit, ~2μg per chromatin immunoprecipitation
Quality
Chromatin Immunoprecipitation:
Sonicated chromatin prepared from HeLa cells (1 X 106 cell equivalents per IP) were subjected to chromatin immunoprecipitation using 2 µg of either a normal mouse IgG, or Anti-dimethyl-Histone H3 (Lys4) antibody and the Magna ChIP G Kit (Cat. #17-611). Successful immunoprecipitation of dimethyl-histone H3 (Lys4) associated DNA fragments was verified by qPCR using ChIP Primers GAPDH Coding (Please see figures).
Please refer to the EZ-Magna G ChIP (Cat. #17-409) or EZ-ChIP (Cat. #17-371) protocol for experimental details.
Sonicated chromatin prepared from HeLa cells (1 X 106 cell equivalents per IP) were subjected to chromatin immunoprecipitation using 2 µg of either a normal mouse IgG, or Anti-dimethyl-Histone H3 (Lys4) antibody and the Magna ChIP G Kit (Cat. #17-611). Successful immunoprecipitation of dimethyl-histone H3 (Lys4) associated DNA fragments was verified by qPCR using ChIP Primers GAPDH Coding (Please see figures).
Please refer to the EZ-Magna G ChIP (Cat. #17-409) or EZ-ChIP (Cat. #17-371) protocol for experimental details.
Target description
Dimethyl-histone H3 at ~17 kDa
Physical form
Anti-dimethyl-Histone H3 (Lys4) (mouse monoclonal IgG1, clone CMA303). One vial containing 50 μg of protein G purified antibody in 50 μL PBS containing 0.05% sodium azide. Store at -20°C.
Normal Mouse IgG. Two vials containing 25 μg purified Mouse IgG in 25 μL storage buffer containing 0.1% sodium azide. Store at -20°C.
ChIP Primers GAPDH Coding. One vial containing 75 μL of 5 μM of each primer specific for the coding region human GAPDH. Store at -20°C.
FOR: GGC TCC CAC CTT TCT CAT CC
REV: GGC CAT CCA CAG TCT TCT GG
Normal Mouse IgG. Two vials containing 25 μg purified Mouse IgG in 25 μL storage buffer containing 0.1% sodium azide. Store at -20°C.
ChIP Primers GAPDH Coding. One vial containing 75 μL of 5 μM of each primer specific for the coding region human GAPDH. Store at -20°C.
FOR: GGC TCC CAC CTT TCT CAT CC
REV: GGC CAT CCA CAG TCT TCT GG
Format: Purified
Storage and Stability
Stable for 1 year at -20°C from date of receipt.
Aliquot upon initial thaw, avoid freeze thaw cycles.
Aliquot upon initial thaw, avoid freeze thaw cycles.
Analysis Note
Control
Includes negative control mouse IgG antibody and control primers specific for human β-globin promoter.
Includes negative control mouse IgG antibody and control primers specific for human β-globin promoter.
Legal Information
UPSTATE is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class
10 - Combustible liquids
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Hong Chen et al.
Cell cycle (Georgetown, Tex.), 17(13), 1602-1613 (2018-06-14)
Small RNAs play an important role in gene regulatory networks. The gene suppressive effect of small RNAs was previously the dominant focus of studies, but during the recent decade, small RNA-induced gene activation has been reported and has become a
Arpana Verma et al.
Journal of cellular biochemistry, 116(5), 743-753 (2014-12-17)
In this study genome-wide di-methylated H3K4 (H3K4me2) and tri-methylated H3K27 (H3K27me3) modification profiles were analyzed in spermatozoa of buffalo bulls having wide fertility differences. The custom designed 4 × 180 K buffalo (Bubalus bubalis) ChIP-on-chip array was fabricated by employing array-based sequential hybridization
Ya-Li Nie et al.
Drug metabolism and disposition: the biological fate of chemicals, 45(12), 1372-1378 (2017-10-14)
Human UDP-glucuronosyltransferase 1A1 (UGT1A1) is a unique enzyme involved in bilirubin conjugation. We previously characterized the hepatic expression of transcription factors affecting UGT1A1 expression during development. Accordingly, in this study, we characterized the ontogenetic expression of hepatic UGT1A1 from the
Qing Huan et al.
The New phytologist, 219(4), 1373-1387 (2018-08-01)
Vernalization, the requirement of plants for long-term exposure to low environmental temperature for flowering, is an epigenetic phenomenon. Histone modification regulation has been revealed in vernalization, but is limited to key genes. Now, we know that VRN1 is epigenetically critical
Fuzhen Qi et al.
Journal of hematology & oncology, 10(1), 48-48 (2017-02-18)
Long noncoding RNAs (lncRNAs) have emerged as important regulators of tumorigenesis and cancer progression. Recently, the lncRNA AGAP2-AS1 was identified as an oncogenic lncRNA in human non-small cell lung cancer (NSCLC) and its elevated expression was linked to NSCLC development
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico