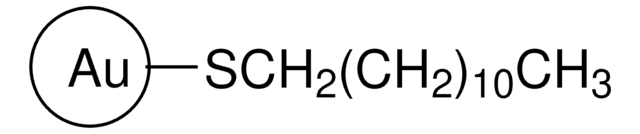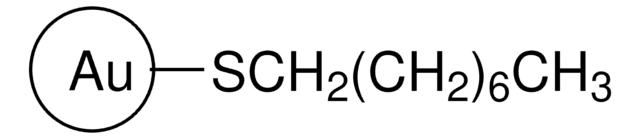These Gold Nanoparticles are prepared at a concentration of 9.08 × 10⁻⁸ M or 90.8 nM. Please refer to the image below for additional parameter:
741949
Gold nanoparticles
5 nm diameter, OD 1, stabilized suspension in citrate buffer
Sinónimos:
Au NP, Gold Colloid
Seleccione un Tamaño
Seleccione un Tamaño
About This Item
Productos recomendados
Nivel de calidad
Formulario
nanoparticles
suspension
contiene
stabilizer (Proprietary Surfactant)
concentración
~5.5E+13 particles/mL
DO
1
diámetro
5 nm
λmáx.
510-521 nm
PDI
<0.2
temp. de almacenamiento
2-8°C
cadena SMILES
[Au]
InChI
1S/Au
Clave InChI
PCHJSUWPFVWCPO-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Descripción general
Aplicación
This material is highly monodisperse (<12% variability in size and shape), and provides significantly improved surface reactivity. Applications include Surface Enhanced Raman Lables, Sensing/Detection, Biological Targeting, Plasmonics and Electronics.
Información legal
Código de clase de almacenamiento
12 - Non Combustible Liquids
Clase de riesgo para el agua (WGK)
nwg
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Steven J. Oldenburg, Ph.D. provides an overview of lateral flow diagnostic assays and discusses the use of ultra-bright reporter particles based on the unique optical properties of gold nanoshells that significantly increase the sensitivity of lateral flow immunoassays.
Sustainable energy sources with high production efficiency are crucial for meeting increasing energy demand.
Gold (Au) nanoparticles have tunable optical and electronic properties and are used in a number of applications including photovoltaics, sensors, drug delivery & catalysis.
Professors summarize recent 2D materials synthesis advancements and biosensing applications in various fields.
-
What is the molar concentration of gold nanoparticles?
1 answer -
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
-
What is extinction coefficient of this gold nano particle?
1 answer-
The Molar Extinction Coefficient for this product is reported to be 1.10E+07 (M-1cm-1).
Helpful?
-
-
How do you attach gold nanoparticles to glass?
1 answer-
To view the procedure on how to attach gold nanoparticles to glass please view information in the reference - Journal of Atomic, Molecular, and Optical PhysicsVolume 2012 (2012). Article ID 683830, 6 pagesdoi:10.1155/2012/683830
Helpful?
-
-
How do you attach gold nanoparticles to peptides and peptide conjugates?
1 answer-
To view information on how to attach gold nanoparticles to peptides and peptide conjugates, please view the information in the reference - Rosenthal, S. J. and Wright, D. W. (Eds.). (2005), p. 91-92. NanoBiotechnology Protocols. New Jersey. Humana Press.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
Which gold nanoparticle size should I choose for my application?
1 answer-
The size of gold nanoparticle to use is very depandent upon the intended application. Generally, smaller particles offer better sensitivity in applications such as immunogold labeling due to less steric hindrance and the ability to bind more gold nanoparticles to the desired target. Small gold nanoparticles are less visible than larger particles, however, which must also be taken into account.As an application example, particles with a size between 30-50nm are particularly useful for the development of rapid tests such as lateral flow assays.
Helpful?
-
-
Why does my gold nanoparticle solution turn violet when I add salt containing buffer?
1 answer-
Due to repulsive forces arising from the surface charge of gold nanoparticles, an energy barrier must be overcome for individual particles to interact. When no (or small) amounts of electrolytes such as NaCl is present, this energy barrier is too strong for interaction to occur between particles. However, upon addition of NaCl this energy barrier is reduced allowing the gold nanoparticles to interact and aggregate. This aggregation causes a phenomenon called surface-plasmon coupling which changes the adsorption maximum of light to a higher wavelength resulting in a change in color of the solution.
Helpful?
-
-
Do I need to wash my gold nanoparticles?
1 answer-
For most applications, our gold nanoparticles can be used without any additional washing steps. If you have a sensitive application that requires additional washing the best way to do so is by either centrifugation or filtration.
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico